Abstract
Leukemia is a type of cancer that affects the blood and bone marrow. Bromodomain and Extra-Terminal (BET) proteins are a family of proteins that regulate gene expression. Despite significant advancement in research, detection and treatment of BET mediated leukemia is a formidable challenge. Aptamers are single-stranded DNA or RNA molecules that bind specifically and tightly to target molecules and are often called “chemical antibodies.” The destruction of BET protein is facilitated by a chemical compound known as PROTAC. We hypothesize that the aptamers used in this research can interact with the BET protein and help diagnose it. Then, chemical mutations help design more potent PROTACs, which then degrade the BET protein. We employed computational techniques including molecular simulations to get insights into the function of BET proteins. Molecular docking simulates the binding of the PROTAC to both the target protein and the E3 ligase to identify the most favorable binding modes and calculate the binding energies that reflect the strength and stability of these interactions. Protein-Ligand Interaction Profiler (PLIP), a computational tool was used to analyze interactions between proteins and ligands that provided insights into how ligands bind to their target proteins. Based on our analysis, Aptamer-1 was selected as the most appropriate candidate. Customizing drug molecules through targeted modifications enhances treatment efficacy and helps minimize off-target effects improving patient outcomes while reducing toxicity. This research hopes to offer a promising, personalized approach to leukemia therapy aligning with the evolving landscape of precision medicine.
Keywords: PROTAC, Molecular docking, Aptamer, Leukemia.
Introduction
Leukemia is a kind of blood cancer affecting the bone marrow and white blood cells. BET proteins, especially BRD4, play a crucial role in regulating gene expression, genes that are involved in cell cycle control, growth, and differentiation.1 These proteins act as readers of epigenetic markers and bind to acetylated lysines on histones, which are proteins that help organize DNA. This binding helps facilitate the expression of genes that drive the growth of leukemia cells.1
BRD4 is a special type of cancer protein that binds to the acetylated lysine residues on histones near the promoter and enhancer regions of the MYC gene.2 It recruits transcriptional machinery, including RNA Polymerase II, and facilitates transcriptional elongation, leading to sustained expression of MYC.3 Overexpression of MYC results in uncontrolled cell division, a hallmark of cancer. Therefore, these proteins are critical targets of anti-cancer therapy. Aptamers have been widely used to diagnose cancer proteins due to their high specificity and affinity. We hypothesize that the aptamers used in this research can interact with the BET protein and help diagnose it. In the current work, we used computational simulations to model the 3D structure of aptamers and then docked them onto the BET protein. Based on docking simulations, we have found that aptamer binds to the bromodomain of the BRD4 protein and thus can prevent leukemia. The current research will help design an Aptamer that can target oncogenic BRD4 protein and potentially prevent cancer progression.4 The BET (Bromodomain and Extra-Terminal domain) protein family consists of BRD2, BRD3, BRD4, and BRDT, all of which function as epigenetic readers that bind acetylated lysine residues on histone tails.5 These proteins contain two conserved N-terminal bromodomains (BD1 and BD2) that recognize acetylated histones, along with an extra-terminal (ET) domain that mediates interactions with other transcriptional regulators. Through this binding, BET proteins help regulate chromatin structure and gene expression. BRD4, a key BET family member, has a unique C-terminal domain (CTD) in addition to its BD1, BD2, and ET domains. This CTD allows BRD4 to interact with P-TEFb, a complex that releases paused RNA polymerase II and promotes transcriptional elongation. BRD4 is crucial in controlling the expression of oncogenes such as MYC and plays an important role in inflammation, cancer progression, and other diseases.2

An aptamer is a short, single-stranded DNA or RNA molecule that can selectively bind to a specific target, such as proteins, small molecules, or even cells, with high affinity and specificity.4 Due to their versatility, aptamers are widely used in biomedical applications, including diagnostics, targeted drug delivery, and biosensing. Their ability to recognize and bind to disease-related biomarkers makes them valuable tools in cancer therapy, infectious disease detection, and precision medicine. Aptamers are generated through a process called Systematic Evolution of Ligands by Exponential Enrichment (SELEX) and function similarly to antibodies but offer advantages like lower immunogenicity, easier synthesis, and higher stability.6 This iterative method begins with a large library of random single-stranded nucleic acid sequences. These sequences are exposed to a target molecule, allowing those with an affinity to bind.6 Unbound sequences are removed, and the bound ones are isolated and amplified using techniques like PCR. This selection and amplification cycle is repeated multiple times to enrich the pool with high-affinity aptamers. Once identified, the aptamer sequences can be chemically synthesized for various applications. Aptamers have a wide range of applications in diagnostics, therapeutics, and research due to their ability to bind specific targets with high affinity.7 In diagnostics, they serve as recognition elements in biosensors and assays, detecting various molecules, including proteins and small compounds. Therapeutically, aptamers can inhibit target proteins, as seen with pegaptanib (Macugen), an FDA-approved aptamer for age-related macular degeneration. They are also being explored for targeted drug delivery and controlled release systems. In research, aptamers facilitate biomarker discovery and are used in imaging applications. Their ease of synthesis, stability, and low immunogenicity make them versatile tools across these fields.8
Aptamers are short, single-stranded nucleic acid sequences, typically comprising 20–100 bases, that fold into unique three-dimensional structures, enabling them to bind specific targets with high affinity and specificity.9 Their secondary structures, such as stem-loops, bulges, and pseudoknots, contribute to the formation of complex tertiary configurations essential for target recognition. For instance, an RNA aptamer targeting the S8 protein adopts a helical structure with several non-canonical base pairs, undergoing significant conformational changes upon binding. Similarly, a DNA aptamer specific to PTK7 forms a three-way junction structure, where distinct helical regions are organized to facilitate target interaction. These intricate structures are central to the aptamer’s function, allowing precise molecular recognition in various applications. In this research paper, we have conducted computational simulations of an aptamer that can target and interact with the BET protein. By targeting the BET protein, it can serve as a biomarker for blood cancer, and the findings of this study may contribute to the detection of blood cancer. The current research will pave the way for the development of novel diagnostic approaches by leveraging the BET protein as a biomarker for blood cancer, potentially improving early detection and treatment strategies.
Method
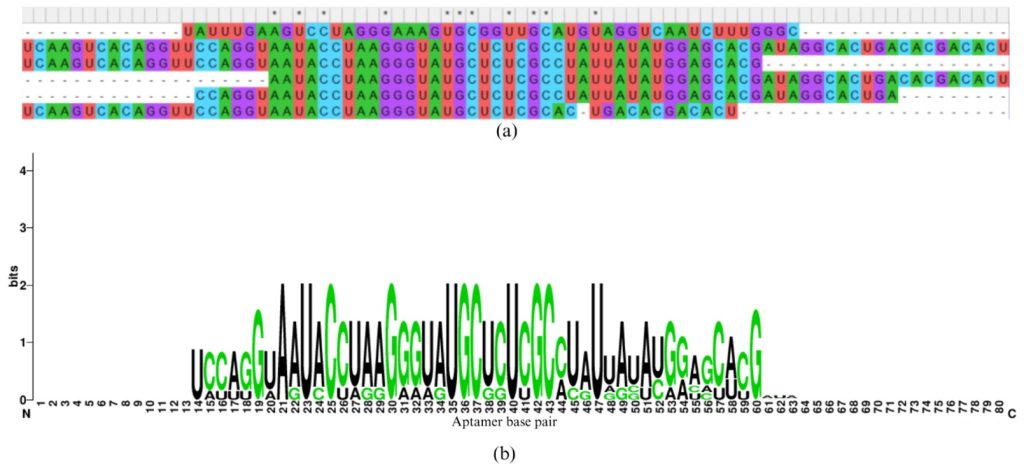
The 3D structure of the BET protein was downloaded from the protein data bank (PDB ID= 5315). Aptamer modeling is a modeling technique that involves predicting the 3D structure of an aptamer from its nucleotide sequence and understanding how it interacts with target molecules, such as proteins, small molecules, or ions. Aptamers are short, single-stranded DNA or RNA sequences. They can fold into unique structures to bind with high affinity and specificity to their targets, making them powerful tools in biotechnology, diagnostics, and therapeutics. In the next step, we need to understand how these aptamers bind to the protein obtained from the Protein Data Bank.10 Therefore, we must model the 3D structure of these aptamers based on the steps outlined below. This is a crucial step because the 3D structures of these aptamers are not available in the Protein Data Bank due to their high flexibility. As a result, we must computationally model these structures. The software used in this process, discussed below, has been experimentally validated. The aptamer modeling case contains the following steps: First, the aptamer sequence is obtained from a previous study and the sequences are shown in Figure 1, which is also called a primary or 1D structure. These aptamer sequences are converted into a secondary structure called a CT file using UNA fold.11 The CT files were later converted to dot and bracket notation using RNA structure.7 The dot and bracket notation represent the hydrogen bond interaction formed in between the aptamer base pairs. The dot represents the non-bonding; the bracket represents the hydrogen bond. The dot and bracket notations were converted to the three-dimensional structure in PDB format by using FarFar2 software.12 The complete conversion of the primary to secondary to the tertiary structure of aptamer is shown in Figure 1. The aptamer analysis was performed by using the PLIP webserver.13 The Protein-Ligand Interaction Profiler (PLIP) web server is crucial for understanding how the aptamers chemically interact with the protein 3D structures. The aptamers form different types of interactions, such as hydrogen bonds, hydrophobic interactions, salt bridges, pi-cation interactions, and pi-pi interactions, all of which play key roles in aptamer binding and contribute to their strong binding affinity. Similarly, the sequence similarities of the aptamers are also analyzed, highlighting the exact sequence similarities among different aptamers. In addition, the sequence logo, generated using a web server, depicts the frequency of specific base pairs at each position within the aptamers.
Results
Aptamer Structure Modeling
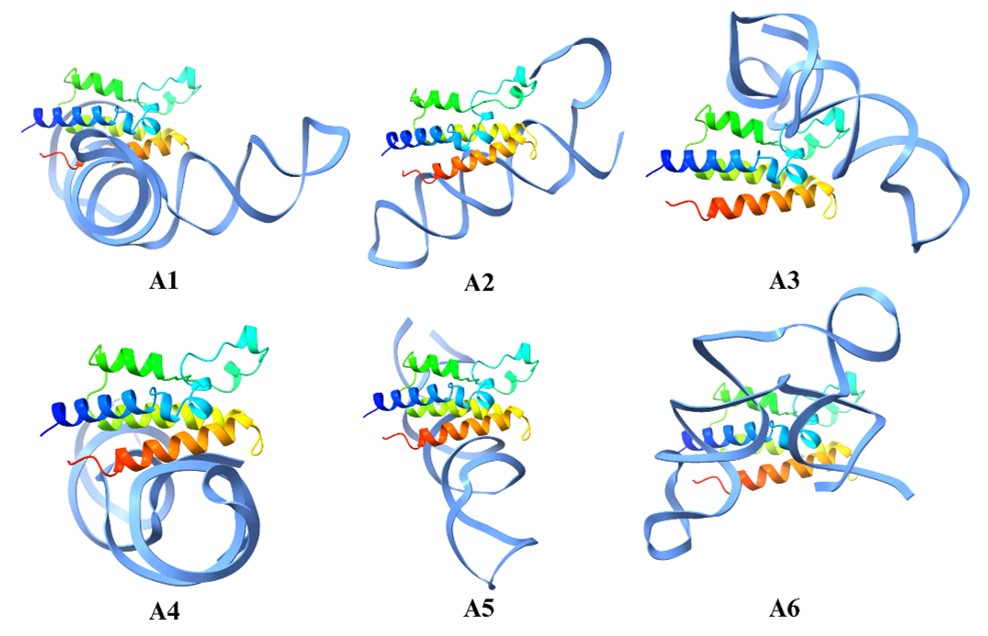
The Aptamer structure modeling was performed, as shown in Figure 2. First, the Aptamer 2D structures were converted to one-dimensional structures; then, they were converted to dots and brackets, also called secondary structures. The 2D structure is shown in Figure 2b, and finally, these structures show the hydrogen bond interactions that were converted into a 3D structure. These structures were later used for molecular docking simulation on the BET protein. This is the linear sequence of nucleotides (A, U, G, C for RNA aptamers or A, T, G, C for DNA aptamers). The secondary structure represents how the aptamer folds based on intramolecular base pairing. Examples: stems (helices), loops, bulges, and hairpins. These structures are important for binding the target molecule. The 3D structure represents the spatial arrangement of the nucleotides. Aptamers form complex shapes that bind their targets with high affinity and specificity. Tertiary interactions include hydrogen bonding, base stacking, and interactions with metal ions or ligands.
Molecular Docking Simulations
In the next step, molecular docking simulation was performed in which the aptamer was docked on the protein structure based on the molecular docking structure as shown in Figure 3. Based on this simulation only aptamer A2, A3, and A6 bind to the predicted binding site and therefore were selected as the most appropriate candidate. Among these aptamers A6 formed the maximum number of interaction (combining both hydrogen bond and salt bridges) and could be an appropriate candidate for binding the protein as shown in Figure 4. Since this aptamer binds strongly to the protein, it can be conjugated with a fluorescent dye and can be used in the detection of cancer. Finally, we have also performed the sequence similarity performed by using MEGA software. In addition, we have also performed sequence LOGO as shown in Figure 5. These two images will help in designing novel aptamers that can be used in the detection of the BET protein.
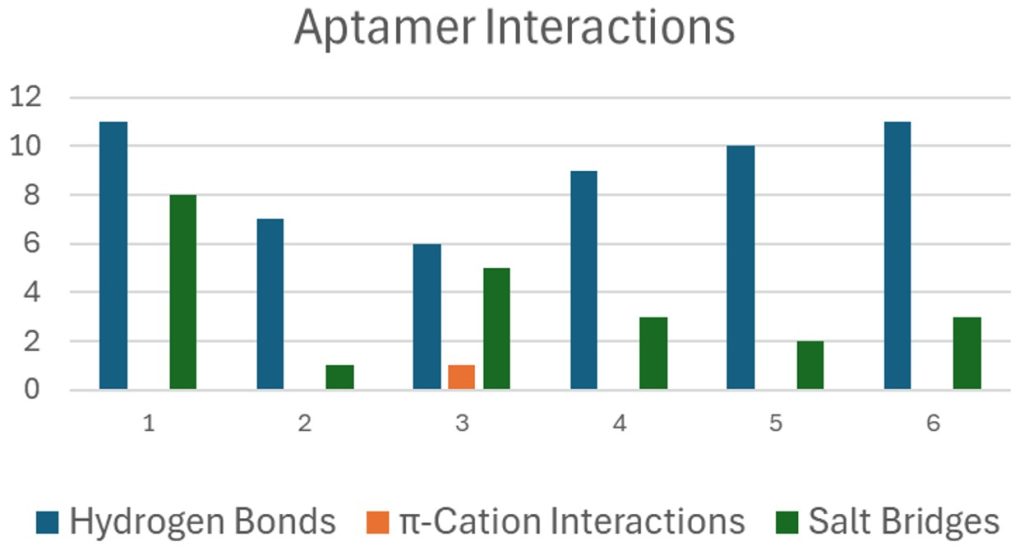
| Aptamer | Docking Score / Binding Energy | No. & Type of Interactions | Key Binding Residues (BRD4) |
| A1 | Not specified (used as reference) | 7 interactions: H-bonds, salt bridges | Lys91, Asp144, Glu97, Tyr98 |
| A2 | Moderate (binds predicted site) | 5 interactions: H-bonds, hydrophobic | Asp144, Glu97, Val87 |
| A3 | Moderate (binds predicted site) | 6 interactions: H-bonds, π-π stacking | Lys91, Tyr98, Phe83 |
| A6 | Best docking score (highest binding energy) | 9 interactions: H-bonds, salt bridges, hydrophobic | Lys91, Glu97, Tyr98, Phe83 |
Discussion
Aptamers bind specifically to cancer-associated biomarkers; hence they can be linked to chemotherapy drugs or nanoparticles to deliver treatment directly to cancer cells. This targeted approach helps minimize damage to healthy tissues, reduce side effects and improve overall treatment effectiveness. Aptamer-drug conjugates (ApDCs) are also being explored as an alternative to antibody-drug conjugates (ADCs), offering advantages like lower immune response and simpler production.
Therapeutic Application
Aptamers bind precisely to disease biomarkers, making them valuable tools in biosensors and imaging techniques like MRI or fluorescence-based detection. Aptamer-coated nanoparticles further enhance the accuracy and sensitivity of diagnostic tests, allowing for earlier and more reliable identification of cancer cells in blood or tissue samples.
Limitations
One of the biggest challenges with aptamers is their instability in biological environments. Enzymes in the body can break them down quickly, limiting their effectiveness. To counter this, researchers often modify aptamers with chemical groups like 2-fluoro or 2-O-methyl to improve their lifespan in the bloodstream. Because of their small size (~10-30 kDa), aptamers are filtered out of the body by the kidneys, which lowers their availability for therapeutic use. To address this, scientists are exploring ways to increase their circulation time, such as attaching larger molecules like polyethylene glycol (PEG) to slow down renal clearance. Aptamers struggle to get through the cell membrane, and this limits their use for treatments that need to work inside cells. Scientists are trying to solve this by linking aptamers to special peptides or using microscopic carriers to help them get inside cells. The process to develop them (called SELEX) requires a lot of work, and making large amounts of them and adding chemical changes can raise costs. Researchers are trying to find cheaper ways to make aptamers more widely available for use in medicine.
Conclusion
This study targets leukemia by inhibiting malignant Bromodomain and Extra-Terminal (BET) proteins by using Aptamers. BET is significant in gene regulation, and its malfunction can lead to cancer. Aptamers are single-stranded DNA or RNA molecules that bind specifically and tightly to target molecules. In this research, we employed computational techniques including molecular simulations, to get insights into the function of BET proteins. Molecular docking helped identify the most favorable binding modes and the binding energies reflecting the strength and stability of these interactions that were then further calculated. Finally, Protein-Ligand Interaction Profiler (PLIP), was used to analyze interactions between proteins and ligands that provided insights into how ligands bind to their target protein. Aptamer-1 was selected as the most appropriate candidate to detect the BET protein. By inhibiting BET protein, our research study demonstrates the potential of developing a cost-effective, targeted and precision-based approach against leukemia.
References
- Juliusson, G., & Hough, R. (2016). Leukemia. Prog Tumor Res, 43, 87-100 [↩] [↩]
- Donati, B., Lorenzini, E., & Ciarrocchi, A. (2018). BRD4 and Cancer: going beyond transcriptional regulation. Molecular Cancer, 17(1), 164. [↩] [↩]
- Venkataraman, G., Song, J. Y., Tzankov, A., Dirnhofer, S., Heinze, G., Kohl, M., . . . Jaffe, E. S. (2013). Aberrant T-cell antigen expression in classical Hodgkin lymphoma is associated with decreased event-free survival and overall survival. Blood, 121(10), 1795-1804 [↩]
- Dunn, M. R., Jimenez, R. M., & Chaput, J. C. (2017). Analysis of aptamer discovery and technology. Nature Reviews Chemistry, 1(10), 0076. [↩] [↩]
- Gadd, M. S., Testa, A., Lucas, X., Chan, K.-H., Chen, W., Lamont, D. J., . . . Ciulli, A. (2017). Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol, 13(5), 514-521 [↩]
- Darmostuk, M., Rimpelova, S., Gbelcova, H., & Ruml, T. (2015). Current approaches in SELEX: An update to aptamer selection technology. Biotechnology Advances, 33(6, Part 2), 1141-1161. [↩] [↩]
- Keefe, A. D., Pai, S., & Ellington, A. (2010). Aptamers as therapeutics. Nature Reviews Drug Discovery, 9(7), 537-550 [↩] [↩]
- Keefe, A. D., Pai, S., & Ellington, A. (2010). Aptamers as therapeutics. Nature Reviews Drug Discovery, 9(7), 537-550. [↩]
- Stojanovic, M. N., de Prada, P., & Landry, D. W. (2001). Aptamer-Based Folding Fluorescent Sensor for Cocaine. Journal of the American Chemical Society, 123(21), 4928-4931 [↩]
- Rose, P. W., Prlić, A., Altunkaya, A., Bi, C., Bradley, A. R., Christie, C. H., Burley, S. K. (2016). The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic acids research, 45(D1), D271-D281 [↩]
- Markham, N. R., & Zuker, M. (2008). UNAFold. In J. M. Keith (Ed.), Bioinformatics: Structure, Function and Applications (pp. 3-31). Totowa, NJ: Humana Press. [↩]
- Watkins, A. M., & Das, R. (2023). RNA 3D Modeling with FARFAR2, Online. In R. K. Kawaguchi & J. Iwakiri (Eds.), RNA Structure Prediction (pp. 233-249). New York, NY: Springer US. [↩]
- Salentin, S., Schreiber, S., Haupt, V. J., Adasme, M. F., & Schroeder, M. (2015). PLIP: fully automated protein–ligand interaction profiler. Nucleic acids research, 43(W1), W443-W447 [↩]