Abstract
Lithium-ion batteries (LIBs) have transformed energy storage, offering high energy density, fast charging, and long cycle life, making them central to electric vehicles and consumer electronics. However, their dependence on finite, ethically problematic, and environmentally harmful resources presents a sustainability paradox. This paper applies a qualitative, literature-based Life Cycle Assessment (LCA) framework to evaluate LIBs holistically across their production, use, and end-of-life stages, beginning with finite and ethically complex raw material extraction and ending with the regenerative recycling of spent Lithium-Ion batteries. Using a qualitative, literature-based methodology, the study examines four major cathode chemistries (cobalt, nickel, manganese-based, and Nickel Manganese Cobalt, NMC) evaluating their structural stability, performance, and orbital interactions. It also explores the role of doping in enhancing cathode efficiency. Post lithium-ion batteries, such as sodium-ion, lithium-sulfur, solid-state, and lithium-air, are reviewed for their potential and current limitations. Additionally, recycling methods including pyrometallurgy, hydrometallurgy, and direct recycling are assessed in terms of emissions, material recovery, and circularity. Results show cobalt-based cathodes offer high energy but poor safety, while manganese variants are safer but less powerful. NMC offers balance, and doping improves overall performance. Among recycling methods, direct recycling is the most sustainable, whereas pyrometallurgy leads to the highest emissions and material loss. The study concludes that material innovation and efficient recycling are both essential to advancing battery sustainability. A systems-level integration of better cathode design and circular economy strategies is critical to reducing LIBs’ environmental impact and enhancing future battery technologies.
Keywords: Lithium-ion batteries, Cathode chemistry, Recycling methods, Post-lithium-ion, Sustainability, Circular economy, Battery limitations
Introduction
Lithium-ion batteries have slowly become the most widely used energy storage system. They have made themselves home in smartphones, laptops, electric vehicles, and even large-scale energy grids, becoming an essential component of modern technology. All lithium-ion batteries are composed of three important elements, including two electrodes: the cathode and anode (positive and negative, respectively) and an electrolyte that separates the electrodes1,2. The performance and efficiency of these batteries depend on the intricate balance of these components—the correct pairings ensure optimal energy storage and discharge. An ideal battery strikes a balance among key factors such as energy capacity, power density, environmental impact, sustainability, renewability, cost, and efficiency, ensuring optimal performance and longevity. Despite current efforts, the search for the perfect battery is still in progress. Mizushima and Goodenough et al. are credited for introducing the Lix-CoO2- Lithium Cobalt Oxide (LCO)3. This battery checked most of the considerations of a perfect cathode: high energy density, good electrical conductivity, high open-circuit voltage (4.0 V), and low self-discharge4. Yet, as time has passed, it has been understood that LCO may not be the sustainable, long-term option the world is looking for due to cobalt scarcity, high costs, ethical concerns, environmental impact, and safety risks such as thermal runaway.
Since the demand for Li-ion batteries is expected to grow exponentially in the coming years, global energy needs for these batteries are projected to increase from approximately 700 GWh in 2022 to around 4.7 TWh by 2030, highlighting the crucial role of understanding how to make better and more sustainable Li-ion batteries for the future of energy storage5.
To be noted, the paper uses 2 units: mAh g⁻¹ and Wh/kg. Specific to Lithium-Ion Batteries Assuming a nominal voltage of 3.7 V, the conversion becomes:
![]()
Methodology
A systematic literature search was conducted using database including, Scopus, Google Scholar and Science Direct. Keywords used to filter literature were, “battery recycling,” “cathode chemistry,” “electrolytes,” and “battery sustainability.”. Filters were applied to limit the results to peer-reviewed journals published from the year 2010 onwards and an impact factor of 5 and greater to ensure both reliability and credibility. The paper uses an equal balance of primary experiment research and review papers based on their relevance to lithium-ion battery technology, environmental implications, materials development, and recycling strategies. To organize the collected studies and references efficiently, Mendeley was utilized, enabling effective management of citations and collection of literature
Raw Material Extraction and Ethical Concerns
Mining, Markets, and Morals
Environmental concerns
The lifecycle of lithium-ion batteries begins with the extraction of critical raw materials such as lithium, cobalt, and nickel. These processes have profound environmental and ethical implications. The paradox of lithium-ion batteries is that they pose significant environmental and ethical challenges. The extraction and refining of raw materials are notable contributors to severe environmental degradation; in fact, the cell production of LIBs also contributes to the already worsening environment. To add to this, the unmanaged hazardous waste at the end of the battery cycle leads to toxic pollution, biodiversity loss, and contamination of water, soil, and air5.
Ethical Concern
The battery value chain also faces significant social and governance challenges that must be managed to ensure ethical and efficient progress. Socially, the mining of raw materials required by different types of Li-ion batteries endangers regional communities. Issues such as violations of labour laws, child and forced labour, and the infringement of indigenous rights are significant risks that need to be addressed. Additionally, the labour involved in the raw material extraction process faces the issue of fair working conditions, proper wages, and protection against discrimination and harassment. These issues are not only ethically challenging but also directly affect the long–term and sustainable viability of lithium batteries, or any type of battery in this respect5.
Future Supply issues
The global demand for the output of precious metals including lithium and cobalt has increased, however, their reserves are limited s the metal composition of spent LIBs has exceeded the content of natural deposits6. Most of the lithium supply is concentrated in countries, including Argentina, Australia, Chile, and China. While the demand for lithium-ion is expected to rise by 95% by 2030, the supply may fall short by 55%5. Similarly, nickel reserves are spread across regions like Australia, Canada, Indonesia, and Russia. The shortage of nickel is less, by 8% compared to demand5. Cobalt, on the other hand, is primarily sourced from the Democratic Republic of Congo (DRC)7 Even though it is predicted that the expectation of cobalt usage in batteries will decrease, supply could exceed demand by 15% (figure 1)5.
Analyzing the predictions made by McKinsey presents an uncertainty of ±10 % in the demand and supply of these valuable metals. It should also be noted that the cited data from McKinsey does not rely only on static assumptions. According to their MineSpans team, quoted “The base-case scenario for raw-material availability in 2030 considers both existing capacity and new sources under development that will likely be available soon.” This demonstrates that the alarming supply shortages (e.g., 55% lithium shortfall) presented in the chart reflect realistic constraints even when considering technological improvements.

Assembling Parts of a Battery
Anode
The anode is a very crucial element of a rechargeable battery, and due to its properties, the anode has a significant effect on the overall performance of the battery8. Graphite, considering their unique hierarchical structure is the most commonly commercially available anode. When lithium-ions insert into graphite spaces, the gaps between the adjacent carbon layers provide perfect insertion sites8. This structure allows for proper anode activity without changes in the anode’s shape, size, and structure during charge and discharge cycles. The anode discharges the lithium-ions into the electrolytes (figure 2)9.

Electrolyte
Electrolytes are a principal component of battery systems they govern the operation of the battery by transporting lithium ions between cathode and anode11. However, a major issue with liquid electrolytes is their instability and high flammability, primarily due to the presence of organic carbonates such as dimethyl carbonate and ethylene carbonate in modern LIBs (figure 2)12.
Cathode
The cathode in a battery enables energy storage and release. During discharge, cathode gains electrons while lithium ions move from the anode through the electrolyte to balance the charge throughout the battery. This movement allows electrical current that powers electrical devices like phones, and EVs. The cathode material determines a battery’s capacity, voltage, lifespan, and overall performance. Common cathode materials in lithium-ion batteries include lithium cobalt oxide (LiCoO₂), lithium iron phosphate (LiFePO₄), and nickel-manganese-cobalt (NMC) oxides (figure 2)13.
Cathode Chemistry
Why Cathodes Matter
The next phase of the LIB lifecycle involves the manufacturing process, particularly cathode formulation, which significantly influences both battery performance and environmental burden. Battery scientists and engineers have been making batteries 5–10% more efficient every year for the past 25 years, says George Crabtree, a materials scientist at Argonne National Laboratory in Illinois14. Yet, cathode materials have become a bottleneck in the improvement and development of better batteries, due to high costs and limitations in electrochemical performance.
Cobalt cathodes were the very first that scientists studied and developed. The capacity of LCO was initially theorized to be 274 mAh g⁻¹, if one mole of Li⁺ deintercalated. However, only half of this theoretical value, approximately 140 mAh, is actually utilized15,16. This inefficiency is caused by the overlap between the Co³⁺/⁴⁺: t₂g and O²⁻: 2p bands. To address this issue concerning cobalt, it has been replaced and tested with other transition metals, aiming to reduce the overlap with the O²⁻ 2p band, thereby improving the efficiency of the cathode material17,16.
Nickel-Based Cathodes
LiNiO₂ (Lithium Nickel Oxide- LNO) was first designed by Dyer in 1954. Its study was later extensively continued by Dahn et al. in the 1990s as a possible replacement for LCO16. Nickel was considered because LNO is isostructural with LCO, and it offers significant improvement in terms of battery efficiency. Unlike LCO, which has efficiency issues due to the overlap between Co³⁺/⁴⁺: t₂g and O²⁻: 2p, LNO involves the eg Band, which lies well above the O²⁻: 2p band18. This reduction in overlap enhances the efficiency by preventing changes in lattice oxygen even at deep deintercalation (is the process of lithium ions exiting the electrode structure during battery discharge) levels of Li⁺18. LNO, hence offers a higher capacity of 200 mAh, approximately 60 mAh above LCO19.
However, LNO still does not fall in the category of an ideal battery (table 1). The LNO battery is highly sensitive to temperature changes. At temperatures higher than that of 250°C, Ni³⁺ becomes unstable, which is issue during the synthesis of “pure” LNO. Under these conditions of high temperatures, unwanted forms of nickel, such as Ni²⁺, can form and occupy spaces that should be filled by lithium ions. This blockage disturbs the movement of lithium ions, reducing the battery’s efficiency. Additionally, high voltages trigger severe stress in the cathode particles, leading to unwanted potential cracking and oxygen release18,20. This reduces the battery’s ability to charge and discharge efficiently.
From an economic and sustainability perspective, nickel remains a concern due to its future availability. By 2030, a small shortage of nickel is projected, with supply potentially falling 8% short of demand5. Therefore, while LNO does possess promising characteristics, it still cannot make the “perfect battery”. Its chemical synthesis challenges and potential nickel supply issues need to be dealt with before making it the leading battery technology.
Manganese-Cathodes
Another widely studied cathode material is lithium manganese oxide (LiMn₂O₄) due to its three-dimensional (3D) crystal structure; this material belongs to the A[B₂] O₄ spinel-type structure (A crystal structure commonly seen in LiMn₂O₄, allowing 3D lithium-ion diffusion)21. This structure is effective because it enables efficient lithium-ion diffusion through a network of interconnected sites18. In this structure, Li+ occupies tetrahedral (8a) sites and Mn3+/4+ occupies the octahedral (16d) sites within a close-packed oxygen lattice. This structure allows Li⁺ to migrate through vacant 8a and 16c sites, constructing a connected 3D network which amplifies the ionic transport within the battery hence improving efficiency18.
While LiMn₂O₄ (Lithium Manganese Oxide- LMO) contains a promising structure, it undergoes significant structural changes during charging and discharging cycles. It is voltage sensitive, at approx. 4.0 V, which means the cubic structure Li⁺ de-intercalates from the 8a sites. However, at approx. 3.0 V, Li⁺ moves in and out of 16c sites, triggering the Jahn–Teller effect caused by Mn³⁺18,22. This effect distorts the structure, changing the structure from a cubic to a tetragonal phase, which leads to rapid capacity loss18,23. One drawback of LMO is Manganese (Mn) dissolution. Ironically, the power-enhancing surface orientation supports Li+ diffusion and is the most sensitive and vulnerable to Mn dissolution which reduces its life cycle24. Making it difficult for LMO to achieve both high power and long life simultaneously. Additionally, just like LNO, LMO also experiences irreversible capacity loss at high temperatures21. This is because, at high temperatures, the phase transitions happening within LMO cause distortions in the structure. Another factor in reducing the battery’s performance is the electrolyte interaction. LMO batteries containing LiPF₆ with traces of HF cause an Mn disproportionation reaction (2Mn³⁺ → Mn²⁺ + Mn⁴⁺). In this reaction, the cathode material dissolves into the electrolyte affecting battery performance21.
These challenges highlight the limitations of LMO, and while it is a significant cathode material for research, further improvements are needed to reduce capacity loss and prepare it for long-term performance. (table 1).
Nickel, Manganese and Cobalt CathodeComposed of varying ratios of nickel, manganese, and cobalt, with higher nickel content, Nickel Manganese Cobalt (NMC) cathodes are widely used in lithium-ion batteries due to their high energy density and stability25. While they do possess high energy density, they exhibit significant electrochemical and structural challenges. Higher nickel content (like in NMC-811) enhances capacity of the battery however reduces thermal stability and battery performance due to phase transitions and electrolyte degradation25. Other issues that this cathode faces include scarcity and high costs and lower electronic conductivity of Manganese, lithium-ion diffusion limitations, voltage fade, and surface reactivity, necessitating coatings and dopants for long-term performance25.
| Comparatives | Lithium Cobalt Oxide | Lithium Manganese Oxide | Nickel Manganese Cobalt Oxide | Lithium Nickle Oxide |
| Abbreviation | LiCoO2 (LCO) | LiMn₂O₄ (LMO) | LiNiMnCoO₂ (NMC) | LiNiO₂ (LNO) |
| Nominal Voltage | ~ 3.6 V | ~3.7–3.8 V | ~3.6–3.7 V | ~3.6 V |
| Full Charge | 4.2 V | 4.2 V | 4.2 V or higher | 4.2 V |
| Full Discharge | ~ 3.0 V | ~3.0 V | ~3.0 V | ~3.0 V |
| Minimal Voltage | ~ 2.5 V | ~2.5 V | ~2.5 V | ~2.5 V |
| Specific Energy | ~140–150 mAh/g | ~100–120 mAh/g | ~150–220 Wh/kg | ~200 mAh/g |
| Thermal Runaway | Risk at ~150 °C | Begins >250°C | ~210°C (high Ni = less stable) | >250°C = Ni³⁺ instability |
| Common Applications | Phones, laptops, tablets | Power tools, medical devices | EVs, E-bikes, medical, grid | High-capacity EVs |
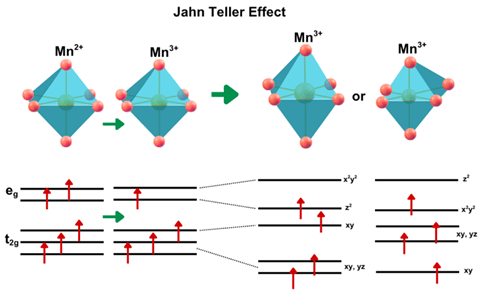
Jahn Teller Effect
The Jahn Teller effect refers to a structural disorientation that occurs in certain transitional metal ion. In lithium-ion batteries, this effect is most evident in cathode materials containing manganese, such as LiNi₀.₈Co₀.₁Mn₀.₁O₂. Mn³⁺ ions. Mn²⁺ starts in a regular octahedral structure (six oxygen ligands equally spaced, figure 3). When Mn²⁺ is oxidized to Mn³⁺, its electron configuration changes which may result in uneven occupancy of the electrons leading to destabilization of the symmetric octahedral geometry causing spontaneous elongation or compression of the Mn2+ octahedra (figure 3).4. Over repeated charge-discharge cycles, the Jahn–Teller effect contributes to lattice degradation, reduced lithium mobility, and ultimately capacity fading. In Ni-rich layered oxides, even a small presence of Jahn–Teller-active Mn³⁺ can negatively impact performance, making it a critical factor in the long-term reliability of lithium-ion batteries.4,27.
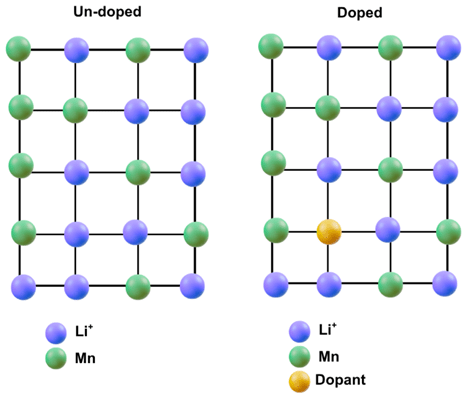
Doping the Future
Ni and Co-based cathodes have a common issue of mixing cations, as Ni²⁺ migrates to Li⁺ sites and vice versa20. This happens due to the similar ionic radii of Ni²⁺ and Li⁺, leading to the migration of Ni²⁺ into empty Li⁺ spots during charging. This process reduces the efficiency of the cathode material, hindering the overall performance28. A solution to this is doping, which involves adding small quantities of foreign elements into the structure of the cathode. These can include Al, Mg, Ti, Zr, Co and more, which replace or fill positions in the cathode that would otherwise get infected by unwanted ions, figure 4.28. Hence, doping helps reduce the migration of Ni²⁺, thereby improving lithium-ion (Li⁺) diffusion and enhancing the overall performance of the cathode. Doping the cathode helps stabilize the cathode structure by suppressing cation mixing and enhancing the structural stability28.
Reaching the Limits of Lithium-ion Batteries
During the operational phase, lithium-ion batteries face technical constraints that affect their lifespan and efficiency, with implications for resource use and safety.
Energy Capacity Constraints
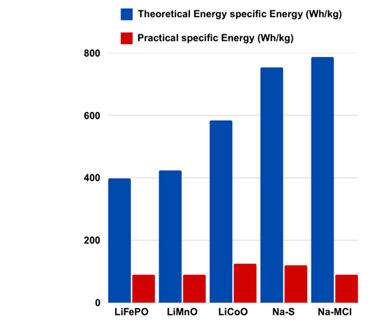
LIBs have offered a promising energy storage solution; however, they fall short of their practical electrochemical capacities compared to their theoretical ones. This is a constraint to achieving long-term energy storage goals that today’s world demands for plug-in hybrid electric vehicles (PHEVs) and electric vehicles (EVs)29. Examples include layered LiMO₂ (M = Co, Ni, Mn), which reaches 140–160 mAh/g, spinel LiMn₂O₄, which achieves 100–120 mAh/g, and olivine LiFePO₄, which falls between 140–160 mAh/g (figure 5)29.
The gap between the theoretical and practical value is prominent across all material in figure 5, this is because the insertion and extraction of Li⁺ (during charging and discharging) strains and fractures the electrode lattices, causing phase changes and oxygen loss. Leading to the breakdown of usual carbonate electrolytes above ~4.2 V.29. Additionally, the use of high-voltage or high-temperature accelerates undesired side reactions at the electrode–electrolyte interfaces. These reasons force designers to under-use active material to keep cells safe and long-lasting. Battery makers don’t let the cells run all the way up to their absolute maximum capacity to avoid overheating, avoid premature wear in order to increase cell safety and lifetime.29.
Possibles solutions to close the gap between theoretical and real-world battery include coating electrodes with thin, lithium-conductive ceramic or polymer layers and using non-flammable, high-voltage electrolytes which can help protect surfaces and allow cells to be driven harder.29. Second, switching to high-voltage spinel cathodes which operate at around 4.7 V also provides a noticeable improvement in the gap.29. Third, the use of composite layered cathodes that blend lithium-rich and transition-metal oxides (which activated above 4.5 V) can be used to deliver much higher capacity. Finally, though not yet proven new chemistries such as lithium–sulfur and lithium–oxygen holds the potential to two to five fold gains in energy density, provided challenges like lithium dendrite formation and reactant “shuttling” can be overcome.29
Dendrite Formation
Dendrite formation on anodes is one of the most significant challenges with LIBs. In the process of charging, when Li-ions don’t deposit evenly on the anode surface, it triggers the formation of needle-like crystalline structures called dendrites. These structures can penetrate the separator, increasing the risk of battery failure as well as short circuits. “Carbon can only accept lithium at a given rate. If you try to send lithium [through the battery] too fast [while charging], the lithium doesn’t really go into the graphite, it sticks on the outside. It becomes a safety hazard” stated Nitash Balsara,30 material scientist at the University of California. Additionally, it can also be concluded that smaller batteries are at higher risk, because “The smaller the battery, the easier it is for dendrites to grow all the way across the electrolyte and contact the opposite pole, shorting out the battery” explained John Goodenough14,6.
Safety Issues
Liquid electrolytes
Traditional LIBs use nonaqueous liquid electrolytes. The organic solvents found in the electrolyte are highly flammable and volatile, such as dimethyl carbonate and ethylene carbonate. This property makes them prone to fires and explosions. The organic liquids are also unstable at high voltages and can undergo degradation over time, reducing the life cycle as well as its efficiency of the battery. To add to the issues with liquid electrolytes, it promotes dendrite formation, which pierces the separator and causes short circuits and potential thermal runaway6.
Recycling Batteries: Closing the Loop
At the end of their useful life, lithium-ion batteries must be processed through various recycling strategies to recover critical materials and reduce environmental impact.
Considering the limitations of lithium-ion batteries, it is crucial to recover valuable metals like cobalt, nickel and lithium, which not only reduce the need for harmful mining practices but also meet the future demand for raw material. Additionally, without proper recycling the issue of the proper waste disposal of materials such as fluorinated electrolytes and heavy metals that are flammable and toxic can contaminate the soil and water, risking serious environmental damage, recycling will conserve resources, lower carbon emissions and support the economy through a circular model, by allowing the waste of today to be the sustainable products of the future.
Mechanical Damage
Indentation and impact that causes mechanical damage to the batteries possess safety concerns, particularly in the application of electric vehicles. Significant deformation of up to 5mm may did not record a major change in the voltage and temperature of the battery making damage detection difficult through conventional electrical monitoring.31.
Electrochemical Impedance Spectroscopy (EIS) and modeling using a distributed Equivalent Circuit Model (ECM) were used to detect subtle changes in internal parameters, but results showed minimal differences between damaged and intact cells. This shows that moderate mechanical damage might not affect battery performance or safety in the short term. However, relying only on voltage or EIS data is insufficient for damage detection. An undetected indentation may remain in the cell and cause a failure when a small additional load moves the deformation to short circuit limits. Hence, advanced detection tools to detect mechanical damage are needed to avoided long term safety issues.31.
Overcharging
Overcharging lithium-ion batteries is directly proportional to fire hazards. The more overcharged as battery gets the higher the changes are for it to catch fire. This is because with over charging the cut-off voltage rises, batteries store and release more energy, leading to higher initial discharging voltages and longer discharge durations.32 It also leads to an increase of surface temperatures due to increased internal resistance and heat generation, exciting intense exothermic reactions. Overcharged cells exhibit earlier vent cracking, ignition, and thermal runaway. Overcharged LIB possesses a more serious combustion process and a lower stability than the normal LIB and a battery with higher cut-off voltage will experience a more violent combustion if ignited. Additionally, the overcharging of batteries also leads to structural damage like casing deformation, electrolyte leakage, and electrode carbonization, ultimately causing short circuits and irreversible battery failure.32
Thermal Runaway
Beyond environmental concerns, LIBs have the issue of thermal runaway and battery fires which is a major safety risk. Issues like these have been costly to humanity: one notable case being Dell’s 2006 recall of 4.1 million Sony batteries due to fire hazards, resulting in an estimated loss of $300 million33
LiNi₀.₈Co₀.₁₅Al₀.₁₅O₂ (NCA) batteries are still used despite their safety concerns, especially concerning thermal runaway. Thermal runaways concern with the internal heat generation surpass the battery’s ability to properly diffuse heat, triggering a temperature rise. These high temperatures cause structural instability by accelerating cathode degradation above 200°C, leading to oxygen release and further heat generation, creating a self-sustaining cycle of rising temperatures. According to Differential Scanning Calorimetry data, NCA undergoes a highly exothermic reaction between 200°C and 250°C, releasing 941 J g⁻¹26 Additionally, phase transitions in the NCA cathode weaken the material’s strength and the presence of Ni⁴⁺ ions at high lithium extraction levels leading to electrolyte decomposition and flammable gas release. Despite these safety concerns, NCA batteries are still at high demand due to their high energy density of 275 mAh g⁻¹ and operating voltage of 4.3 V (vs. Li⁺/Li⁰)26.
lithium-ion battery (LIB) fire incidents are increasing with the push toward higher energy densities. There is a proven trade-off between energy density and safety is well-documented and thermodynamically grounded.26. This occurs due to granter energy storage in smaller volumes, which risks the changes of thermal runaway as oxygen releases from high voltage cathodes. Additionally, flammable electrolyte decomposition at elevated temperatures.26.
Other real-world examples include that of Tesla Model S fires (a significant case being that of the 2016 incident in Oslo and other being the 2017 one in Shanghai) which highlighted the dangers of high-energy-density cells using Ni-rich cathodes like NCA.26. Second, The Boeing 787 Dreamliner fleet was also grounded in 2013 due to battery fires from lithium cobalt oxide cells26.
Battery Recycling
Pre-treatment
The recycling of the LIBs involves critical pretreatment steps to efficient and safe metal extraction in the recovery processes. The pretreatment stage involves sorting, discharging, dismantling, shredding, and mechanical-physical separation34. At the beginning of the process, used LIBs are manually sorted and discharged to below 0.5V to avoid potential risks such as fire or explosion risks during shredding. Salt-saturated solutions including NaCl and Na2SO4 are used for discharge; however, these solutions may trigger complex reactions in which electrolytes can leak into the salt solution6.
In the next step, mechanical shredding takes place in an inert atmosphere purified with argon or nitrogen, where LIBs are crushed using duo-directional shredder blades. The inert gases help prevent lithium from reacting violently with oxygen which otherwise may lead to combustion34.
The objective of pre-treatment is to effectively disassemble the important components of the battery. This makes recycling more efficient, reduces waste, and simplifies the subsequent steps of the recycling process (figure 5).
Pyrometallurgy
This process includes the heating of metal oxides present in the battery. Batteries that have undergone pre-treatment are heated in an inert atmosphere, to transform metal oxides into a mixture of metals and alloys. The compounds recovered from this process depend on the type of battery and typically include cobalt, nickel, copper, iron, and slag containing lithium and aluminum35. While this method does allow for the recycling of LIBs, it is most effective for other valuable metals including cobalt35.
The process includes putting pre-treated LIB components into a large furnace, turning them into valuable metal alloys. Developing businesses including Umicore are using this as their primary form of battery recycling in fact only certain batteries require disassembly before the process of pyrometallurgy takes place36. This recycling method allows to close of the material loop in the battery value chain Sommerville, R. et al. A qualitative assessment of lithium ion battery recycling processes. Resour Conserv Recycl 165, (2021).
However, some components are lost in the process, as electrolytes, plastics, and graphite burn. Additionally, Al, Li and Mn are not recovered and are lost in the form of slag due to high temperatures. Research to extract Li from this formation of slag is in the processes, refer to figure 437,38.
Hydrometallurgy
This recycling method uses aqueous solutions to extract and separate metals from LIBsSommerville, R. et al. A qualitative assessment of lithium ion battery recycling processes. Resour Conserv Recycl 165, (2021). The valuable cathode material is dissolved in acid and separated using solvent extraction. Pre-treated batteries undergo extraction, using H2SO4, H2O2 and other organic compounds35. This method is growing in popularity due to its efficiency and ability to recycle more parts of a LIB. Hydrometallurgy method guarantees high recovery rates of valuable metals (figure 6)6.
However, plastics, casings, current collectors, and graphite do not get recycled in the process of hydrometallurgy. Additionally, in the long run it is not feasible for companies to opt for this due to the immensely detailed process and high cost of the high volume of acid and base required for leaching6,39.
6.1.4 Direct Recycling
Direct recycling is a process that includes the direct removal of cathode material for reuse or reconditioning. It is more efficient than classical methods since it recovers still-working cathode particles without them breaking down into different components or getting wasted or precipitated40. The step after battery pre-treatment includes froth flotation, which is a method that separates compounds from cathode material depending on their property of hydrophobic and hydrophilic. When put into water, different compounds float or sink depending on these characteristics allowing for easy separation. However, the presence of residual Polyvinylidene Fluoride (PVDF) can impact the efficiency of the process if given thermal treatment. It should be noted that efficiency of froth flotation is highly dependent on both the chosen characteristics of the specific cathode material. Cathode composition and surface chemistry can how separation occurs flotation, hence, cathodes with different chemistries require customized pretreatment and flotation conditions, as the hydrophobicity and binder adherence differ across formulations as the presence of (PVDF) differs across all cathoods. After separation, the recovered powders undergo acid leaching for purification, a process that has demonstrated high efficiency in recovering valuable metals41. Since direct recycling requires the proper disassembly of each material in a battery the complex and diverse nature of LIBs has hindered the adoption of automated disassembly solutions. These inconsistent battery designs has led to difficulty in making a stadarized system of recycling force this method to be labour intensive. Due to the potential hazards such as chemical leaks, thermal runaway, and exposure to toxic materials in the process of direct recycling, worker safety must be prioritized.
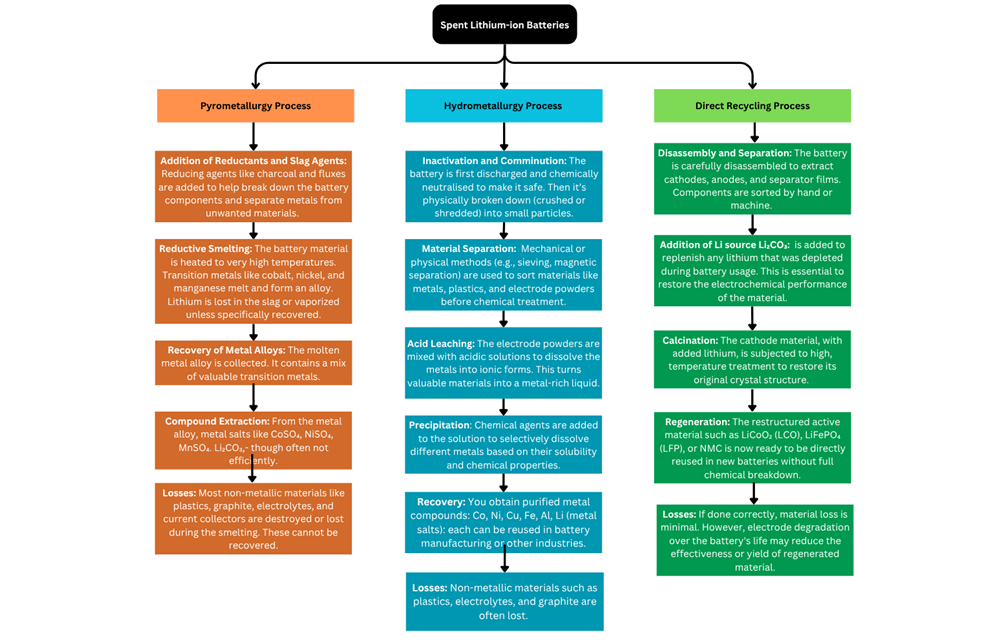
Comparison of the recycling methods
CO₂ Emissions from Recycling Processes Pyrometallurgy shows the highest CO₂ emissions (~10 kg CO₂e kg / battery) for all 3 batteries. This is expected because this process is dependent on high temperatures for the recycling of valuable material contributing to high CO2 emissions (figure 6).
Hydrometallurgy has lower emissions than pyrometallurgy across all battery types. This is because the process involves acid leaching which does not require the need for high temperatures hence reducing energy consumption and emissions. Direct recycling has the lowest emissions among the three processes and hence seems to be the most environmentally friendly approach. (table 2) Since direct recycling just preserves cathode materials without requiring chemical or heating processes, it is the least destructive environmentally.

| Pyrometallurgical | Hydrometallurgical | Direct Recycling | |
| Process | Uses high temperature smelting to recover metals. | Uses aqueous solutions to dissolve and extract metals. | Recovers and Redevelops cathode materials for reuse. |
| Efficiency | Effective for recovering Co, Ni, Cu, and Fe. | High recovery rates of transition metals. | Preserves cathode structure, reducing the need for new materials. |
| Material Loss | Li, Al, and Mn often lost in slag. | Non-metallic components (plastics, graphite, electrolyte) are not recovered. | Minimal material loss; retains cathode structure. |
| Environmental Impact | High CO₂ emissions (~10 kg CO₂e per kg battery). | Lower emissions than pyrometallurgy. | Lowest emissions; more sustainable. |
| Cost & Viability | Profitable for Co/Ni-rich LIBs; widely used. | Costly due to acid use; starting commercialization. | Still limited in scale and automation. |
Economic feasibility and scalability of recycling methods
The economic feasibility and scalability of different recycling methods vary significantly. Firstly, pyrometallurgy, is compatible with mixed battery types is costly due to high energy consumptions and low material recovery efficiency, figure _. However, when the battery being recycled contains a large amount of cobalt, a very valuable metal, the money earned from recovering that cobalt can cover or exceed the costs of the process, making it economically worthwhile. If the battery has little or no cobalt, the process becomes less profitable or even unfeasible. Hence, making it economically viable only for cobalt-rich batteries.35.
Secondly, hydrometallurgy, gives better recovery rates compared to that of pyrometallurgy, (figure _) however the process involves significant chemical and wastewater treatment costs, which affect the profitability unless cobalt content is high. Since the costs of hydrometallurgy exceed that of pyrometallurgy scalability of this recycling methods is still not grand and companies tend not to use this due to lower profitability.43,42.
Lastly, direct recycling has the lowest estimated processing cost of around $6 per kilogram for NMC/NCA. However, it is not yet scalable due to the need for standardized battery formats and automation in disassembly and sorting. Even though direct recycling offers the most cost-effective method of recycling its scalability remains an issue.42. It should also be noted that, due to the low recycling cost and high value ofcathode production, the direct recycling could create a profit of 1.71 billion dollars, nearly double that of metallurgy-based recycling.41.
| Recycling method | Estimated Cost | Main Economic Factors | Feasibility |
| Direct Recycling | ~$6/kg (NMC/NCA cathode) Virgin cathode cost: ~$21–24/kg | Low-costavoids refining metalsretains material value | Most cost-effective, but low scalability |
| Hydrometallurgy | Not fixed, varies by process due to variation in plant design, reagents, and recovery targets. However, to be noted it has higher operating costs than direct recycling due to acid and base usage and wastewater treatment. | High chemical and treatment costsviable only with high cobalt and/ or nickel content | Moderate scalability, economically sensitive |
| Pyrometallurgy | High costs due to: High-temperature operations which are often >1,000°CLoss of low-value metals like lithium and aluminium | Profitable only with cobalt-rich batteries as lithium and aluminum is often lost in slag. | Scalable, but least efficient and expensive |
Immerging and green recycling methods
Bioleaching
As an eco-friendly methods bioleaching stands as a battery recycling method that uses microorganisms including bacteria and fungi to leach valuable metals like lithium, cobalt, and nickel from spent lithium-ion batteries. These bacteria and fungi produce acids and other compounds through their metabolism that dissolve metals from the shredded battery material, known as black mass. While other methods use high temperatures and harsh chemicals, bioleaching operates under mild and natural conditions which makes the process safer and more sustainable.
The process of bioleaching includes bacteria like Acid thiobacillus ferroxidase. These thrive in acidic environments. Additionally it also uses fungi such as Aspergillus niger function in near-neutral conditions.
The nature of the process is slow and sensitive to factors such as pH, temperature and metal toxicity however it can be enhanced using microbial consortia, adaptation techniques, and even ultrasound. Hence, bioleaching offers a low energy, low emission alternative to traditional recycling which also addresses the circular economy goals.
Waste-for-Waste (W4W)
Waste-for-Waste (W4W) is a sustainable recycling approach. It includes the use of discarded food waste as a natural reductant to extract valuable metals from spent lithium-ion batteries. This process uses common food wastes like tea leaves, grape seeds, and orange peels which are rich in compounds such as polyphenols, flavonoids, and reducing sugars. These work effectively to dissolve metals like lithium and cobalt when combined with mild acids like citric or malic acid. This method replaces hazardous chemicals like hydrogen peroxide with safer, biodegradable alternatives derived from waste, making in highly sustainable and aligned to the SDG goals. Hence significantly reducing environmental impact and operational risks.34.
Electrochemical methods
Electrochemical methods rely on controlled redox reactions to recover valuable metals form spent lithium-ion batteries. Instead of relying on chemical reductants like hydrogen peroxide, these methods apply an external electric current to drive metal dissolution and recovery processes.
Leaching assisted by electrochemical reactions metals such as cobalt and manganese are extracted from battery black mass into solution using acid, with the electric current enhancing reaction efficiency and reducing chemical consumption.
Importantly, selective metal recovery is achieved through “electrodeposition”. This is when metals are reduced and plated onto cathodes based on their standard reduction potentials. While lithium cannot be directly electrodeposited from aqueous solutions due to its highly negative potential, other metals like cobalt, nickel, and copper can be selectively recovered by adjusting parameters such as pH, current density, and electrode material. Electrochemical recycling is not only environmentally friendly but also offers precise control over what and how much of a product is wanted.
The Circular Economy Revolution: Rethinking Battery Lifecycles
The circular economy approach to battery supply chains aims to minimize waste generation and enhance resource efficiency by keeping materials in continuous use for as long as possible. Unlike the traditional linear model of “make, use, dispose”, the circular model emphasizes reuse, refurbishment and recycling, thereby reducing the demand for virgin raw materials and mitigating environmental impact. This approach also presents significant economic opportunities for businesses engaged in battery manufacturing and refurbishment.
The process begins with virgin material, such as Li, Co and other essential metals and resources, which are mined and extracted from various countries. In a linear system, these materials are extracted, manufactured, utilized and ultimately discarded. However, as illustrated in the circular economy model for batteries (figure 8), regenerated materials can be reintegrated into the supply chain, thereby reducing reliance on mining and minimizing waste. Notably, the battery recycling is expected to play a critical role in the broader metal recovery value chain, with global revenues projected to exceed $95 billion annually by 204044.
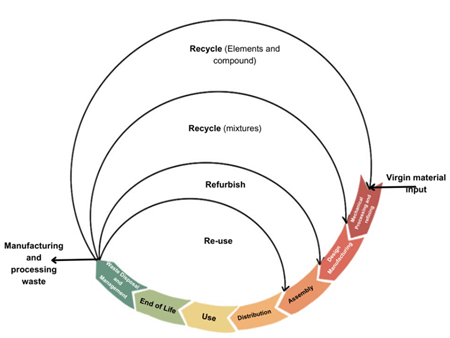
Post Lithium-Ion Battteries
Despite their widespread adoption, LIBs face several challenges, including high cost, limited energy density, and resource constraints. Additionally, the LIB’s market is projected to grow exponentially at approximately 27% annually, reaching around 4,700 GWh by 2030, further straining already limited resources46. This encourages researchers to explore alternatives to LIBs such as sodium-ion batteries (SIBs), solid-state batteries (SSBs), lithium-sulfur batteries (LSBs), and lithium-air batteries (LABs). These technologies offer advancements to LIBs including the potential for higher energy density and lower cost.
Sodium-Ion Batteries (SIBs)
Sodium-ion batteries (SIBs) are considered one of the most direct alternatives to LIBs due to their shared similarities. Leading the way are Na1.5VPO4.8F0.7 and Na4Co3(PO4)2P2O7 which show electrochemical performance comparable with that of the best Li-ion cathodes47. However, the challenge lies in developing high-performance anode materials. Hard carbon, for example, experiences a capacity loss of about 300 mAh g⁻¹ at low current rates and has poorer electrochemical activity compared to lithium-ion batteries (LIBs)47. Additionally, since Na⁺ ions have a larger atomic weight and ionic radius than Li⁺ ions, specialized structural designs are necessary for sodium-ion batteries48. More research needs to be done to find these optimum specialized structures. In times of lithium shortages, however, SIBs can serve as a practical alternative39.
Solid-State Batteries (SSBs)
Composed of solid electrolytes instead of liquid electrolytes, SSBs represent major technological advancements. This change enhances safety by reducing the risk of fire and allows for the use of lithium metal anodes, which can significantly boost energy density. In an ideal case, a solid-state electrolyte plays the role of an ionic conductor and electron insulator by being mixed in the active cathode material slurry, it also suppresses lithium metal dendrite formation and growth, enhancing battery safety, longevity, and overall performance39. However, just like every other battery, solid state batteries also have issues with dendrite formation. Specific to SSB, the poor contact between the electrodes and solid electrolyte reduced the performance, while the high manufacturing costs hinder long-term stability and stable commercialization.
Lithium-Sulfur Batteries (LSBs)
This technology uses nanostructured sulfur/carbon composites with a high amount of conductive carbon as the anode and lithium as the cathode. It also heavily relies on a soluble polysulfide species electrolyte39. LSBs have been theorized to have a high energy density of around >400 Wh/kg. However, the high amounts of electrolyte required also reduce the practical energy density making it a key issue in LSBs39.
Lithium-Air Batteries (LABs)
In comparison to SIBs, SSB and LSB, LABs differ fundamentally due to the active use of a gaseous cathode material, oxygen39. LABs have the highest theoretical energy density of all PLIBs, as they use oxygen from the air as a cathode material, reducing battery weight49. While this may sound like a promising emerging technology, it also faces technical challenges. This is due to the atmospheric gases in the air (N2, CO2 or H2O). If these come in contact with other parts of the battery, unwanted chemical products can be formed in the cathode, which consequently impacts the cycle of the battery. These challenges make LABS, the least commercially viable alternative.
| Technology | Energy density | Cost | Scalability |
| Sodium-Ion Batteries (SIBs) | Lower than Li‐ion ≈100–160 Wh/kg | Relatively low | High as it builds on existing Li‐ion technology |
| Solid-State Batteries (SSBs) | Very high theoretical ~350–500 Wh/kg | Very high as it requires pure O₂, and additional catalysts | Low as it has complex cell design and O₂ which required intensive management |
| Lithium-Sulfur Batteries (LSBs) | Moderate–high ≈200–300 Wh/kg | High as it uses solid electrolytes | Moderate as production lines and emerging |
| Lithium-Air Batteries (LABs) | High theoretical ~350–550 Wh/kg, practical ~250–300 Wh/kg | Moderate, while sulfur is abundant the cathode design needed has higher costs. | Moderate with development still underway. |
Conclusion
This study provides a comprehensive life cycle perspective on lithium-ion batteries (LIBs). LIBs have become indispensable to modern energy systems due to their high energy density and versatility, however their dependence on finite and ethically problematic resources, combined with safety risks and environmental consequences. Through evaluating various cathode chemistries, electrolyte behavior, recycling processes, and post-LIB innovations, this paper shows that the pathway to a more sustainable battery future demands on innovation.
The key findings of the research highlight that no single cathode chemistry achieves an ideal balance across all performance metrics, the incorporation of doping strategies presents a promising avenue to enhance cathode stability and performance across chemistries. Additionally, direct recycling emerges as the most environmentally and economically sustainable option among existing recycling methods, this position stands due to its low emissions and potential for cathode preservation, though it remains limited by scalability. These finding shed light on the urgency of fixing issues with existing batteries, dealing with the future demands and supply needs of raw material and set up a systematic, feasibly, and scalable recycling methods.
Importantly, the objective of this paper is to critically assess LIB technology through a life cycle assessment. The analysis extends to the unexpected challenges within the life of a Li-ion Battery such as the hidden emissions and inefficiencies within certain recycling methods, as well as the economic dependence on cobalt-rich batteries for profitability, thermal runaway, dendrite formation and more. These insights show that LIB sustainability is a challenge that has to be dealt with the earliest.
From these finding its can be recommended that, scientists and researchers aim their time and resources to set a proper recycling method, that is standardized and sustainable. It must also be made sure it is economically feasible and viably without hurt the environment. Current methods who useful have tradeoffs and not one method that is sustainability and economically feasible at the same time. Hence in the tong-term a shift toward a circular economy must be accompanied by cross-sector collaboration to ensure both environmental preservation and energy resilience.
The paper collects research from a wide base of peer-reviewed literature and credible sources. However, to some reliance on existing data limit its ability to fully predict emerging trends. Nonetheless, as the paper transparently addresses these limitations, its spotlights the importance of ongoing work and innovation in the field of Li-ion batteries.
At the end, achieving a sustainable battery future depends not only on perfecting materials but also on redesigning systems.
References
- Winter, M., Barnett, B. & Xu, K. Before Li Ion Batteries.Chemical Reviews vol. 118 11433–11456 (2018). [↩]
- Kim, T., Song, W., Son, D. Y., Ono, L. K. & Qi, Y. Lithium-ion batteries: outlook on present, future, and hybridized technologies. Journal of Materials Chemistry A vol. 7 2942–2964 (2019). [↩]
- Goodenough, J. B. & Manivannan, V. Cathodes for Lithium-Ion Batteries: Some Comparisons. (1998). [↩]
- Kim, T., Song, W., Son, D. Y., Ono, L. K. & Qi, Y. Lithium-ion batteries: outlook on present, future, and hybridized technologies. Journal of Materials Chemistry A vol. 7 2942–2964 (2019). [↩] [↩] [↩] [↩]
- Fleischmann, J. et al. Battery 2030: Resilient, Sustainable, and Circular. https://www.mckinsey.com/industries/automotive-and-assembly/our-insights/battery-2030-resilient-sustainable-and-circular (2023). [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Du, K., Ang, E. H., Wu, X. & Liu, Y. Progresses in Sustainable Recycling Technology of Spent Lithium-Ion Batteries. Energy and Environmental Materials vol. 5 1012–1036 (2022). [↩] [↩] [↩] [↩] [↩] [↩]
- Gulley, A. L. China, the Democratic Republic of Congo, and artisanal cobalt mining from 2000 through 2020. Proc Natl Acad Sci U S A 120, (2023). [↩]
- Qi, W. et al. Nanostructured anode materials for lithium-ion batteries: Principle, recent progress and future perspectives. J Mater Chem A Mater 5, 19521–19540 (2017). [↩] [↩] [↩]
- Blomgren, G. E. Liquid electrolytes for lithium and lithium-ion batteries. in Journal of Power Sources vols 119–121 326–329 (2003). [↩]
- Winter, M., Barnett, B. & Xu, K. Before Li Ion Batteries. Chemical Reviews vol. 118 11433–11456 (2018). [↩] [↩]
- Park, J., Kim, K. & Kang, K. Expanding the diversity of lithium electrolytes. Nature Chemistry vol. 16 1390–1391 (2024). [↩]
- Song, Z. et al. A reflection on polymer electrolytes for solid-state lithium metal batteries. Nat Commun 14, (2023). [↩]
- Caleb Omata Ilabija et al. Advances in nanomaterials for lithium-ion batteries: Enhancing energy density and lifespan. World Journal of Advanced Engineering Technology and Sciences 13, 560–588 (2024). [↩]
- Eric C. Evarts. Lithium batteries: To the limits of lithium. Nature (2015). [↩] [↩]
- Farahmandjou, M. et al. Oxygen redox chemistry in lithium-rich cathode materials for Li-ion batteries: Understanding from atomic structure to nano-engineering. Nano Materials Science vol. 4 322–338 (2022). [↩] [↩]
- Xie, Y., Jin, Y. & Xiang, L. Li-rich layered oxides: Structure, capacity and voltage fading mechanisms and solving strategies. Particuology 61, 1–10 (2022). [↩] [↩] [↩]
- Farahmandjou, M. et al. Oxygen redox chemistry in lithium-rich cathode materials for Li-ion batteries: Understanding from atomic structure to nano-engineering. Nano Materials Science vol. 4 322–338 (2022). [↩]
- Nasajpour-Esfahani, N. et al. Comprehensive review of lithium-ion battery materials and development challenges. Renewable and Sustainable Energy Reviews vol. 203 (2024). [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Song, Z. et al. A reflection on polymer electrolytes for solid-state lithium metal batteries. Nat Commun 14, (2023). [↩]
- Darbar, D. et al. An Overview of Cobalt-Free, Nickel-Containing Cathodes for Li-Ion Batteries. (2022). [↩] [↩]
- Julien, C. M., Mauger, A., Zaghib, K. & Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics (Basel) 2, 132–154 (2014). [↩] [↩] [↩]
- Song, J. et al. Building Better Full Manganese-Based Cathode Materials for Next-Generation Lithium-Ion Batteries. Electrochemical Energy Reviews vol. 6 (2023). [↩]
- Song, J. et al. Building Better Full Manganese-Based Cathode Materials for Next-Generation Lithium-Ion Batteries. Electrochemical Energy Reviews vol. 6 (2023). [↩]
- Kim, J. S. et al. A truncated manganese spinel cathode for excellent power and lifetime in lithium-ion batteries. Nano Lett 12, 6358–6365 (2012). [↩]
- Greenwood, M., Wentker, M. & Leker, J. A region-specific raw material and lithium-ion battery criticality methodology with an assessment of NMC cathode technology. Appl Energy 302, (2021). [↩] [↩] [↩]
- Mauger, A., Julien, C. & Mauger, A. Critical review on lithium-ion batteries: are they safe? Sustainable? [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Li, Shuzhen & Zhang, Hao & Liu, Yong & Wang, Li & He, Xiangming. Comprehensive Understanding of Structure Transition in LiMnyFe1−yPO4 during Delithiation/Lithiation. Advanced Functional Materials. (2023). [↩] [↩]
- Susai, F. A. et al. Improving Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Materials for Lithium-Ion Batteries by Doping with Molybdenum-Ions: Theoretical and Experimental Studies. ACS Appl Energy Mater 2, 4521–4534 (2019). [↩] [↩] [↩]
- Thackeray, M. M., Wolverton, C. & Isaacs, E. D. Electrical energy storage for transportation – Approaching the limits of, and going beyond, lithium-ion batteries. Energy and Environmental Science vol. 5 7854–7863 (2012). [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Eric C. Evarts. Lithium batteries: To the limits of lithium. Nature (2015). [↩]
- Soudbakhsh, D. et al. Electrical response of mechanically damaged lithium-ion batteries. Energies (Basel) 13, (2020). [↩] [↩]
- Ouyang, D., Liu, J., Chen, M. & Wang, J. Investigation into the fire hazards of lithium-ion batteries under overcharging. Applied Sciences (Switzerland) 7, (2017). [↩] [↩]
- Mauger, A., Julien, C. & Mauger, A. Critical review on lithium-ion batteries: are they safe? Sustainable? [↩]
- Roy, J. J. et al. Green Recycling Methods to Treat Lithium-Ion Batteries E-Waste: A Circular Approach to Sustainability. Advanced Materials vol. 34 (2022). [↩] [↩] [↩]
- Baum, Z. J., Bird, R. E., Yu, X. & Ma, J. Lithium-Ion Battery Recycling─Overview of Techniques and Trends. ACS Energy Lett 7, 712–719 (2022). [↩] [↩] [↩] [↩] [↩]
- Sommerville, R. et al. A qualitative assessment of lithium-ion battery recycling processes. Resour Conserv Recycl 165, (2021). [↩]
- Sommerville, R. et al. A qualitative assessment of lithium ion battery recycling processes. Resour Conserv Recycl 165, (2021). [↩]
- Bae, H. & Kim, Y. Technologies of lithium recycling from waste lithium-ion batteries: A review. Materials Advances vol. 2 3234–3250 (2021). [↩]
- Duffner, F. et al. post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nature Energy vol. 6 123–134 (2021). [↩] [↩] [↩] [↩] [↩] [↩]
- Sloop, S. et al. A direct recycling case study from a lithium-ion battery recall. Sustainable Materials and Technologies 25, (2020). [↩]
- Wei, G. et al. Direct recycling of spent Li-ion batteries: Challenges and opportunities toward practical applications. iScience vol. 26 (2023). [↩] [↩]
- Ciez, R. E. & Whitacre, J. F. Examining different recycling processes for lithium-ion batteries. Nature Sustainability 2, 148–156 (2019). [↩] [↩] [↩] [↩] [↩]
- Baum, Z. J., Bird, R. E.,Yu, X. & Ma, J. Lithium-Ion Battery Recycling─Overview of Techniques and Trends. ACS Energy Lett 7, 712–719 (2022). [↩]
- Breiter, A., Linder, M., Schuldt, T., Siccardo, G. & Vekić, N. Battery Recycling Takes the Driver’s Seat. https://www.mckinsey.com/industries/automotive-and- assembly/our-insights/battery-recycling-takes-the-drivers-seat (2023). [↩]
- Velázquez-Martínez, O., Valio, J., Santasalo-Aarnio, A., Reuter, M. & Serna-Guerrero, R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries vol. 5 (2019). [↩]
- Fleischmann, J. et al. Battery 2030: Resilient, Sustainable, and Circular. https://www.mckinsey.com/industries/automotive-and-assembly/our-insights/battery-2030-resilient-sustainable-and-circular (2023). [↩]
- Walter, M., Kovalenko, M. V. & Kravchyk, K. V. Challenges and benefits of post-lithium-ion batteries. New Journal of Chemistry 44, 1677–1683 (2020). [↩] [↩] [↩]
- Sawicki, M. & Shaw, L. L. Advances and Challenges of Sodium-Ion Batteries as Post Lithium Ion Batteries.www.rsc.org/advances. [↩]
- Wu, Z. et al. Evolving aprotic Li-air batteries. Chemical Society Reviews vol. 51 8045–8101 (2022). [↩] [↩]
- Sawicki, M. & Shaw, L. L. Advances and Challenges of Sodium Ion Batteries as Post Lithium Ion Batteries. www.rsc.org/advances. [↩]
- Duffner, F. et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nature Energy vol. 6 123–134 (2021). [↩]