Abstract
Multiple sclerosis (MS) is an incurable, autoimmune disease of the central nervous system, affecting over 2.3 million patients globally, manifested by fatigue, spasticity, vision problems, tremor, and disability. Research suggests correlation of MS to the variations in the earth’s magnetic field, hence cuprizone exposed planarians were treated with low dose pulsating electromagnetic field (PEMF) in attempts to reverse neurological damage, thereby providing a potential treatment option for MS. Planaria were selected due to the structural similarity of their nervous system to the human nervous system, and cuprizone, a copper-chelating agent, was used since it induces toxic demyelination, mimicking the effects of multiple sclerosis. By recording the number of head whips before and after treatment and comparing them with the non-treatment arm, the researcher found that PEMF was significantly able to reverse cuprizone-induced-damage in the planarians after 3 days of treatment, indicating that it may be an effective treatment option for MS. There was a significant effect of PEMF in the ethanol control arm, showing that PEMF could be investigated further as a treatment of alcohol induced neurotoxicity.
Keyword: Planaria, PEMF, Cuprizone, Multiple Sclerosis, Demyelination
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disorder in which the immune system attacks the protective covering, myelin, around nerve fibers. It affects the central nervous system, including the brain, spinal cord, and optic nerves, and can lead to complications in the brain communicating with the rest of the body. The National Multiple Sclerosis Society estimates that there could be about 1 million cases of MS in the U.S. alone out of the 2.3 million diagnosed cases.1. Although there is currently no definitive way to accurately predict the exact progression of MS nor directly correlate it to a single cause, MS has been found to have geo-temporal association, with strong positive correlation between prevalence of MS and distance from the equator, suggesting a strong role for environmental factors which vary with latitude, especially ultraviolet radiation and vitamin D.2. MS is also more prevalent in females and in people with class I and class II alleles of the major histocompatibility complex (MHC), particularly the HLA-DRB1 locus.3. There has also been association with human pin worm infection and Epstein Barr Virus infection.4. Finally, there is a inverse correlation between global MS prevalence and the horizontal component of the geomagnetic field. Research suggests that earth’s magnetic field protects all living beings from harmful effects of cosmic radiation, hence in areas of a weak horizontal magnetic field, there is less mitigation of the ionizing radiation, increasing the risk for developing MS.5. MS is classified into four categories: clinically isolated syndrome (CIS), relapse-remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS).6. Likewise, MS currently obtains no cure; however, there are treatments available to control or delay neurodegeneration resulting from the disease. Such treatments include disease-modifying agents (interferons, glatiramer, fingolimod, teriflunomide, alemtuzumab, etc.), anti-inflammatory agents (methylprednisolone and prednisone), and behavioral therapy.7. Interferon beta was the first disease modifying therapy that was approved to treat MS. There are four different kinds of interferon, and they have been shown to decrease relapse in RRMS and prevent the onset of disability. However, these are all injectables and can cause flu like symptoms and injection site reactions8. Glatiramer acetate is an immunomodulating drug, prepared as an analogue of myelin basic protein and found to inhibit demyelination, and prevent progression and disability in RRMS and PPMS9. However, it can cause major side effects of rash, injection site reaction, lipoatrophy, anaphylactic reaction, shortness of breath, chest pain and hepatic toxicity.10. However, many current treatments for MS have limited efficacy and are associated with significant side effects, highlighting the need for novel therapeutic approaches. Pulsed electromagnetic fields (PEMFs) have been proposed as a potential treatment to reduce plaque size, decrease recurrence, and possibly halt the progression of MS.11.
Nearly every cell in the human body is sensitive to electromagnetic fields, particularly those in the nervous system, due to the active flow of charged ions through membrane protein channels. Additionally, electromagnetic fields influence hormone secretion from the pineal gland, which is sensitive to both light and magnetic fields. Dysregulation of the pineal gland has been hypothesized as a contributing factor to demyelination.12. PEMFs operate at cellular and physiological levels, enhancing the function of cells, including those impaired by disease processes like MS.13. They hold the potential to improve the quality of life for MS patients while avoiding the side effects commonly associated with chemical treatments. Although the precise mechanisms by which electromagnetic fields influence the nervous system remain unclear, several hypotheses have been proposed. These include modulation of charged ion flow through membrane protein channels, enhancement of signal conduction in dysfunctional neurons, and improvement of neuroimmune chemistry disrupted by MS. In this study, the researcher plans to investigate the effects of PEMFs at varying intensity levels to better understand their therapeutic potential and optimize treatment conditions for MS.
Planaria are free-living flatworms belonging to the phylum Platyhelminthes within Kingdom Animalia. Their nervous system includes a centralized brain and a pair of ventral nerve cords, making them one of the simplest organisms to exhibit a true brain structure, which shares functional similarities with the human nervous system. Biochemically, they obtain neurotransmitters and receptors found in mammalian vertebrates, such as acetylcholine, catecholamines, and serotonin. Moreover, planaria are sensitive to many stimuli including texture, vibrations, electric fields, magnetic fields, light, and gravity. By analyzing the planaria’s responses to various stimuli, insights can be gained into the potential effects of neurotoxins on human behavior.14. Two-dimensional tracking is a reliable method for monitoring the activity of planaria, allowing the measurement of various parameters such as velocity, turn frequency and direction, pathways, contractions, and immobilization.15. Among the different planarian species, Dugesia species is particularly suitable for studying the effects of magnetic fields16. Furthermore, planarians are cost-effective and easy to handle, making them an ideal model organism for laboratory studies. PEMFs are generated by accelerating electric charges, combining electric fields from stationary charges and magnetic fields from moving charges. These fields propagate at the speed of light and can interact with the nervous system, potentially inducing behavioral changes17. At both the cellular and physiological levels, PEMFs have been shown to enhance the function of affected cells, including those impaired by disease processes such as MS. They also hold promise for improving the quality of life in MS patients without the side effects typically associated with chemical treatments. Although the precise mechanisms by which electromagnetic fields influence the nervous system remain unclear, several hypotheses exist. These include modulating the flow of charged ions through membrane protein channels, enhancing signal conduction in dysfunctional neurons, and improving neuroimmune chemistry disrupted by MS.18. Researchers have postulated planarians being a potential model for multiple sclerosis research as exhibited by the differences in their behavior, for cuprizone treated planaria exposed to patterned magnetic field. Planarians were exposed to varying concentration of cuprizone and treated with magnetic field or no magnetic field. It was found that exposure of 50 nT, 7 Hz magnetic field, simulated a sudden geomagnetic storm, and reversed the impaired planaria behavior induced by cuprizone.19. There has been extensive research on the capacity of Pulsed Electromagnetic Fields (PEMFs) in influencing vibration of free ions on cell membrane causing alteration of the signal transduction pathway and affecting cellular behavior.20. There are different biological responses depending upon the strength, amplitude and duration of exposure of the PEMF. We used PEMF of strength 80nT to mimic earth’s geo-magnetic storm which ranges from 50-100nT.21. Low frequency PEMF of 20Hz was used as they are thought to be particularly effective because they closely mimic the earth’s natural electromagnetic field, where the electromagnetic field ranges from 0-30 Hz.22. PEMF device was made that could generate a magnetic field of 80nT at a 20 Hz frequency. The magnetic field pattern used simulated the typical onset of a geomagnetic storm (an impulse) and hence exposed to a duration of 10 minutes.
Cuprizone (bis-cyclohexanoneoxalyhydrazone), is a selective and sensitive copper-chelating agent with a high affinity for copper ions, even at low concentrations.23. Its ability to bind copper ions disrupts essential copper-dependent enzymes, leading to cellular dysfunction. In experimental models, cuprizone induced toxic demyelination that closely resembles the pathological demyelination observed in MS.24. In fact, studies have shown that cuprizone can result in up to 70% demyelination in the central nervous system.25. The demyelination induced by cuprizone is contingent upon continuous exposure; when treatment is halted, spontaneous remyelination occurs. This characteristic makes cuprizone a particularly useful tool for studying both the processes of demyelination and the potential for remyelination. Additionally, cuprizone-induced demyelination is reproducible and localized, making it a reliable and widely used model for investigating the mechanisms underlying demyelination events and evaluating potential therapeutic strategies for MS. Thus, by providing a controlled method for inducing demyelination, cuprizone enables the researcher to explore various aspects of neural injury and recovery, further advancing understandings of MS and other demyelinating diseases.
Question: Can pulsating an electromagnetic field with field strength of 80nT and a frequency of 20 Hz eliminate the effects of cuprizone in cuprizone-treated planaria? If so, how long of treatment is necessary?
Hypothesis
The researcher hypothesizes that the behaviors in the cuprizone-treated planaria will be modified after being exposed to PEMF with a field strength of 80nT and a frequency of 20Hz for 10 minutes for three days.
Materials
- 18 Petri Dishes
- Brown Dugesia Planaria
- Copper coils
- Sandpaper
- Hot Glue Gun
- Dropper
- 1-liter container
- Small air pump
- Connectors
- Multimeter
- PEMF Generator
- 0.15 g Cuprizone
- 100 mL Ethanol (98%)
- 1 L Spring Water
- 2 Boiled Egg Yolks
- Aluminum foil
- Pipettes
- Scale
- Weighing sheet
- 50 mL Beaker
- 3- 100 mL Containers
- I Pad
- Grid Paper.
Procedure
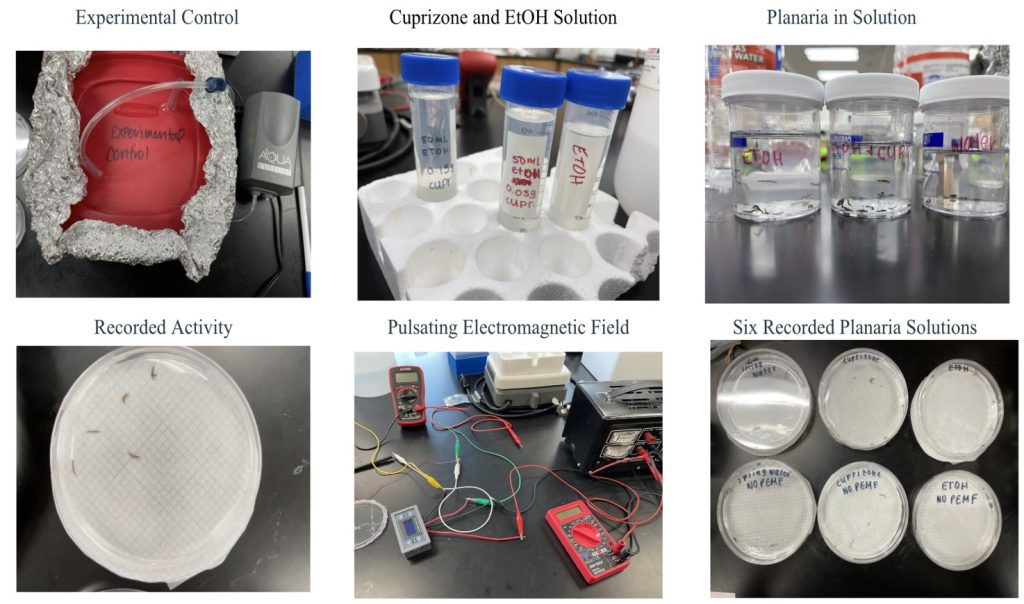
- Planaria were carefully transferred with a dropper from packaging to a one-liter container, half-filled with water, and covered in aluminum foil.
- A small air pump was inserted and the lid of the one-liter container was left slightly open.
- The planaria not used for current testing lived in the one-liter container and were fed egg yolk once a week.
- A copper coil was hot glued around the cap of one petri dish with extra coil left at the ends.
- Insulation was rubbed off with sandpaper, so the copper wire underneath was visible.
- 0.15 grams of cuprizone was measured and dissolved in 50 mL of ethanol.
- In one 100 mL container, 30 planaria were transferred with a pipette along with 0.335 mL of the previous solutions. Spring water was filled to the 100 mL mark.
- In another 100 mL container, 30 planaria were transferred with a pipette along with 0.335 mL of ethanol. Springwater was filled to the 100 mL mark.
- In a third 100 mL container, 30 planaria were transferred and spring water was filled to the 100 mL mark.
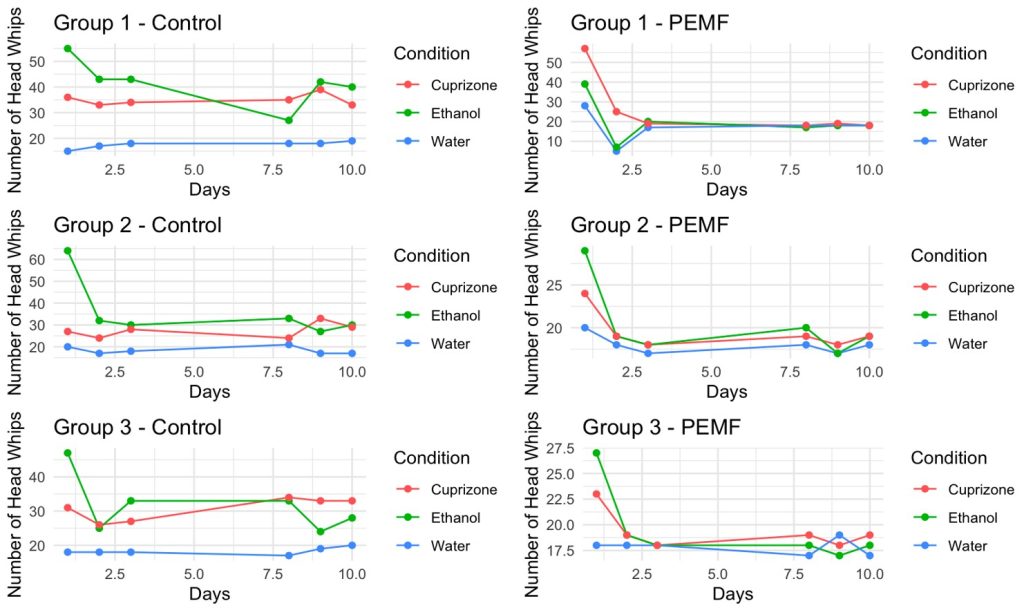
- 24 hours after being submerged in the individual solutions, 5 planaria with their solutions were transferred in a Petri dish lined with graph paper (Day 1). In total, there were 6 Petri Dishes of planaria: spring water, ethanol, and cuprizone with a treatment and a control arm for each (Group 1).
- The PEMF Generator was set with a frequency of 20Hz and a field strength of 80nT.
- Planaria in the treatment arm were treated for 10 minutes at 24 hours (Day 1) 48 hours (Day 2), 72 hours (Day 3), 192 hours (Day 8), 216 hours (Day 9), and 240 hours (Day 10).
- The treated planaria’s behaviors were observed and videotaped with a I pad for 5 minutes after treatment. Then the number of head whips was recorded within the 5-minute period.
- The non-treated planaria’s behavior was observed and videotaped for 5 minutes. The number of head whips was recorded in the time frame.
- Steps 7 – 14 were repeated for Group 2 and Group 3.
- The head whips were compared between the treatment and non-treatment groups.
- One way analysis of variance (ANOVA) was used to calculate the statistical significance and efficacy of the treatment.
Data
Results
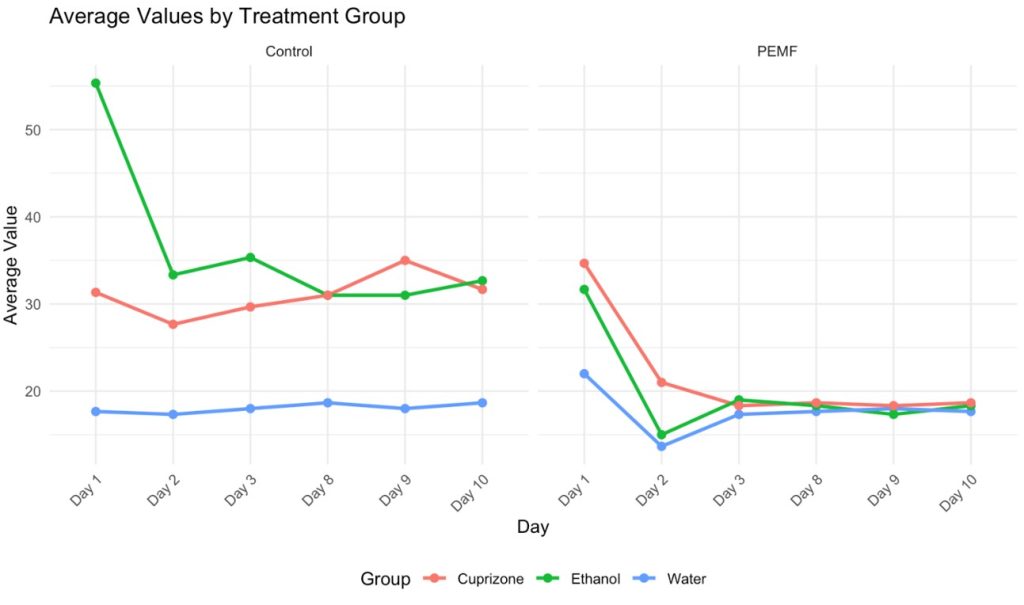
In the absence of treatment, the activity of spring water planaria remained stable, with an average of 18.06 head-whips. The average change in head-whip frequency from day 1 to day 10 was minimal, at just 1 head-whip. In the treatment group, spring water planaria exhibited an average of 17.72 head-whips, resulting in a difference of 4.33 head-whips over the same period. A one-way analysis of variance (ANOVA) was performed to determine statistical significance, and the data were plotted using Excel. With an alpha level set at 0.05, no statistically significant difference was found between the control and treatment groups for spring water planaria, as evidenced by a p-value of 0.7427. Conversely, cuprizone-exposed planaria without treatment exhibited an average of 31.05 head-whips, with a minimal change of 0.34 head-whips from day 1 to day 10. In contrast, treated cuprizone-exposed planaria had an average of 21.61 head-whips, reflecting a substantial average difference of 16 head-whips over the same timeframe. The statistical analysis yielded a p-value of 0.00033, indicating a significant difference between the cuprizone-exposed and treated planaria. Furthermore, planaria exposed to ethanol displayed a marked decrease in head-whip frequency, particularly from day 1 to day 2, followed by a gradual decline thereafter. The untreated ethanol group averaged 36.44 head-whips, with an average difference of 22.67 head-whips observed. In the ethanol treatment group, the average number of head-whips was 19.94, resulting in an average difference of 13.34 head-whips from day 1 to day 10. This group also demonstrated statistical significance, with a p-value of 0.0000036. By day 3, the number of head-whips in the treatment groups for spring water, cuprizone, and ethanol-exposed planaria converged within the range of 17 to 19, which mirrored the head-whip frequencies recorded in the spring water control group.
Conclusions
The hypothesis was supported by the results obtained in this study. Cuprizone-treated planaria exhibited a positive response to pulsed electromagnetic fields (PEMF) with a frequency of 20 Hz and a field strength of 80 nT. Behavioral assessment of planaria, quantified by the frequency of head-whips, revealed an increase to a range of 17-19 over three days in the cuprizone-treated group, which was comparable to the head-whip frequency observed in the spring water control group. The statistical analysis yielded a p-value of 0.00033, indicating that PEMFs were effective in reversing cuprizone-induced damage. Furthermore, the treatment was found to be safe, as the application of PEMF did not result in mortality or harm to the planaria in the spring water control group, with head-whip frequencies remaining stable within the 17-19 range. Additionally, the PEMF treatment effectively reduced head-whip frequencies in planaria exposed to cuprizone dissolved in ethanol, demonstrating a significant p-value of 0.000003, thus suggesting that PEMFs can mitigate the effects of ethanol as well.
Limitations
There are several limitations of this study. Planaria are an excellent model for neurological study, but they lack blood brain barrier, hence it is difficult to generalize the results to human brain and nervous system, where there is blood brain barrier. Cuprizone is not water soluble, hence needed to be dissolved in ethanol, for which ethanol control was used. However, ethanol can also cause neurotoxicity, and the study found that PEMF also affected the ethanol-treated planaria. It is unclear how much effect of ethanol is being counteracted by PEMF versus the effect of cuprizone in cuprizone treated planaria, as they are being exposed to both cuprizone (solute) and ethanol (solvent). Head whips of the planaria were recorded using a Ipad, however since the experiment was performed by one observer; blinding could not be done. The number of head whips were checked 3 times by the observer to avoid observer bias. There was no sham PEMF in this study, but this can be done in future. Also, this study looked at improvement of the planaria behavior as a surrogate to reversal of toxic damage. No dissection was performed to look at the amount of actual demyelination planaria in either arm.
Discussion
The results of these experiments indicated that brief exposures to a neurotoxic agent like ethanol or cuprizone leading to behavioral changes in the planarian can be overcome by short duration of treatment by pulsating electromagnetic field. Changes in the planaria behavior was calculated by their gross movements of head-whips, as seen in the control arm. The behavior was reversed by 3-day PEMF treatment with frequency of 20 Hz and a field strength of 80nT. The statistically significant effect of PEMF on cuprizone treatment arm, was confounded by the statistically significant effect of PEMF on the ethanol control arm, making it difficult to determine if PEMF decreased the neurotoxicity from the cuprizone or ethanol or both. Cuprizone can only be dissolved in ethanol, and not in water, hence the only way to determine the difference is cross comparison of the ethanol and the cuprizone dissolved in ethanol arm. There was no difference in the effect of PEMF between those two arms. Other studies have found that cuprizone causes damage to myelin and gliosis in the planarian, which could be halted by the effect of the patterned magnetic field. Further histological, regenerative and molecular analysis can be done in subsequent studies to study the process of damage done to the planarians by alcohol and cuprizone and learn the process of healing via the patterned magnetic field or even the self-regeneration process possessed by the planarian. We are unable to use this model in vertebrates, as cuprizone does not penetrate the blood brain barrier.
Future Expansions
The effectiveness of PEMF therapy requires further investigation in planarians using a larger sample size, and using histological studies, so we can understand the process of demyelination as well as the process of healing. Cuprizone could be dissolved in lower concentrations of alcohol to take away the confounding factor of alcohol affecting planarian behavior. PEMF therapy can be tested in planarian exposed to ethanol only, and used in animal models, such as vertebrates or primates. If validated, it could emerge as a viable treatment option for patients with alcohol intoxication. Additionally, the appropriate dosing and treatment duration need to be evaluated in larger animals that possess a blood-brain barrier to determine if a higher dose or longer treatment period is necessary. Once the optimal dose and duration for effective PEMF therapy are established in animal studies, Phase 1 clinical trials could be initiated with patients suffering from advanced multiple sclerosis. Moreover, given that PEMFs have shown promise in mitigating the effects of ethanol, they may also be utilized to treat patients experiencing alcohol intoxication, potentially saving lives in cases of overdose. Finally, researchers may examine planarians at the histological, regenerative, and molecular levels for indicators of multiple sclerosis to further substantiate the efficacy of the study.
References
- Brazier, Yvette. Multiple Sclerosis: What you need to know- Medical News Today Article #37556 [↩]
- Simpson S Jr. et al. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg. Psychiatry. 2011 [↩]
- Lincoln MR et al. A predominant role for the HLA class II region in the association of the MHC region with multiple sclerosis. Nat Genet. 2005 [↩]
- Kearns et. Al. Hypothesis: Multiple sclerosis is caused by three-hits, strictly in order, in genetically susceptible persons, Multiple Sclerosis and Related Disorders, Volume 24, 2018, Pages 157-174 [↩]
- Brett Wade et al. Correlations of the Earth’s Magnetic Field Intensity with Global Prevalence of Multiple Sclerosis. American Journal of Life Sciences, 1(2), 31-36 [↩]
- Filippi, Marco. “Multiple Sclerosis.” Nature Reviews Disease Primers, vol. 4, no. 1, 2018 [↩]
- Hauser, Stephen L. “Treatment of Multiple Sclerosis.” American Journal of Medicine, vol. 133, no. 12, 2020, pp. 1380-1390 [↩]
- Mary Filipi- “Interferons in Treatment of Multiple Sclerosis.” International Journal of MS Care, 2019, Oct 30:22(4): 165-172 [↩]
- Tselis A, Khan O, Glatiramer acetate in the treatment of multiple sclerosis. Neuropsychiatry Dis Treat. 2007 Apr; 3(2): 259-67 [↩]
- Glatiramer Injection: Package Insert/Prescribing info from Drugs.com, Feb 2025 [↩]
- Funk, R. H. W. “A Short Review on the Influence of Magnetic Fields on Neurological Diseases.” Frontiers in Bioscience, vol. 26, 2021, pp. 181-18 [↩]
- Sandyk, R. “The Pineal Gland, Cataplexy, and Multiple Sclerosis.” International Journal of Neuroscience, vol. 80, no. 3-4, 1995, pp. 213-220 [↩]
- Pawluk, William. “Healing with Magnetic Field Energy.” Dr. Pawluk, 11 Dec. 2018 [↩]
- Pagan, O. R. “Planaria: An Animal Model That Integrates Development, Regeneration, and Pharmacology.” International Journal of Developmental Biology, vol. 61, no. 1, 2017, pp. 1-10 [↩]
- Neil Deochand- “Behavioral Research with Planaria. Perspective Behavioral Science, 2018, Nov 9;41 (2): 447-464 [↩]
- KA Jenrow et al. “Weak extremely low frequency magnetic fields and regeneration. Bio electromagnetics 1995 [↩]
- Luigi, C. “Mechanisms of Action and Effects of Pulsed Electromagnetic Fields (PEMF) in Medicine” Journal of Medical Research and Surgery, vol. 1, no. 1, 2020 [↩]
- Luigi, C. “Mechanisms of Action and Effects of Pulsed Electromagnetic Fields (PEMF) in Medicine.” Journal of Medical Research and Surgery, vol. 1, no. 1, 2020 [↩]
- Mekers WFT. Introduction of Planaria as a New Model for Multiple Sclerosis Research: Evidence from Behavioral Differences in Cuprizone Treated Planaria Exposed to Patterned Magnetic Fields [↩]
- Mansourian M, Shanei A. Evaluation of Pulsed Electromagnetic Field Effects: A Systematic Review and Meta-Analysis on Highlights of Two Decades of Research In Vitro Studies. Biomed Res Int. 2021 Jul 29;2021:6647497. doi: 10.1155/2021/6647497. PMID: 34368353; PMCID: PMC8342182 [↩]
- Top 50 Geomagnetic Storms- Space weather live.com [↩]
- Catherine Constable, Steven Constable, A grand spectrum of the geomagnetic field, Physics of the Earth and Planetary Interiors. Volume 344, 2023 [↩]
- G. Jean Harry, Arrel D. Toews, “Handbook of Developmental Neurotoxicity” 1998, Chapter 4-Myelination, Dysmyelination and Demyelination pages 87-115 [↩]
- Zhan, Jiangshan. “The Cuprizone Model: Dos and Do Nots.” Cells, vol. 9, no. 4, 2020, p. 843 [↩]
- Torkildsen, Ø. “The Cuprizone Model for Demyelination.” Acta Neurologica Scandinavica Supplementum, vol. 188, 2008, pp. 17-20 [↩]