Abstract
The gut-brain axis (GBA) is a complex communication system that links the gastrointestinal tract to the brain through immune, neural, and hormonal signals. In recent years, it has been shown to play a key role in both brain cancers like glioblastoma and neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis. These conditions, while different on the surface, share common disruptions including chronic inflammation, immune system imbalance, microbial dysbiosis, and blood-brain barrier breakdown. Standard treatments often focus only on the brain and do not address these broader biological issues. This review explores how gut-brain axis dysfunction contributes to disease progression and introduces new treatment strategies that target this system. It highlights innovative tools like engineered probiotics, short-chain fatty acid therapies, gut-enhanced immunotherapies, and gut-guided CAR T cells. A three-tiered model is proposed that organizes therapies into microbiome restoration, immune regulation, and brain protection. While GBA-based strategies show promise across neurological diseases, their effectiveness likely depends on disease subtype, individual microbiome profiles, and timing of intervention. By working across systems, they offer a more precise and personalized way to treat complex diseases.
Introduction
The gut-brain axis (GBA) is a complex communication system between the gastrointestinal (GI) tract and the central nervous system (CNS) that works through neural, immune, and hormonal signals. It is influenced by the gut microbiota, which are microorganisms in the GI tract that help regulate digestion, immunity, and brain function. In recent years, more studies have shown that the GBA plays a role in diseases beyond the digestive system, especially in brain-based cancers and neurodegenerative disorders. Conditions like glioblastoma, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis have all been linked to GBA dysfunction. In glioblastoma, the GBA may affect tumor growth and response to treatment through immune changes and inflammation. In Alzheimer’s and Parkinson’s, problems in the gut microbiome have been connected to increased inflammation and brain cell damage. These patterns suggest that the GBA could be a shared biological factor in how these diseases develop and get worse.
This review explores the question: How does the gut-brain axis affect the development and progression of brain-based cancers and neurodegenerative diseases, and what common biological pathways can be targeted to develop new treatments? The purpose is to find overlapping mechanisms across these diseases, such as inflammation, immune system imbalance, microbial signaling, and blood-brain barrier breakdown, and to evaluate how these might be used to design better therapies. Most existing treatments focus mainly on the brain itself and leave out the bigger picture, which includes gut health and the immune system. By studying the GBA, researchers may discover therapies that are more precise, less invasive, and potentially more effective. This review only includes studies that directly connect the GBA to these diseases and excludes papers not related to shared biological pathways or treatment approaches. No specific theoretical model was used, but the research is based on existing scientific findings related to the gut-brain axis.
Mechanisms of Gut-Brain Axis Dysfunction
One of the earliest changes in gut-brain axis dysfunction is the loss of intestinal barrier integrity, often called “leaky gut.” When the gut microbiome is imbalanced, tight junction proteins between intestinal epithelial cells become disrupted, allowing bacterial fragments and metabolites like lipopolysaccharide (LPS) to leak into the bloodstream. This triggers the release of pro-inflammatory cytokines such as IL-6 and TNF-α. These cytokines not only promote inflammation but also depolymerize cytoskeletal proteins in gut and brain endothelial cells, weakening their structure. As a result, immune cells can cross these compromised barriers in a process known as diapedesis. This combination of gut lining permeability and blood-brain barrier disruption allows inflammatory signals and immune cells to enter the brain more easily, contributing to conditions like multiple sclerosis and Alzheimer’s disease1.
Beyond barrier breakdown, gut microbiome imbalance also disrupts immune signaling. Toll-like receptors (TLRs) on immune cells detect microbial products such as LPS and activate innate immune responses. Chronic stimulation of these pathways skews the balance between regulatory T cells (Tregs), which control inflammation, and T helper 17 (Th17) cells, which promote it.
This results in elevated levels of IL-6, TNF-α, and IL-1β and reduced levels of the anti-inflammatory cytokine IL-10. In a mouse study, Agirman et al. showed that dysbiosis increased brain levels of IL-6 and TNF-α, weakened the blood-brain barrier, and activated glial cells2. These immune shifts create a chronic inflammatory environment that accelerates neurodegeneration and makes brain-based diseases more difficult to treat.
Overview of Neurological Diseases and Current Treatment Limits
Glioblastoma multiforme (GBM), Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS) are major neurological disorders that affect millions of people worldwide and currently have no cures. GBM is an aggressive brain tumor that accounts for nearly 15 percent of all primary brain tumors and about half of all malignant central nervous system tumors in adults. Standard treatments include surgical resection, radiation, and temozolomide chemotherapy, but most patients relapse within a year, and the five-year survival rate remains below 7 percent3. Although immunotherapies like checkpoint inhibitors and CAR T cells are being tested, GBM’s immunosuppressive environment limits their success4. One of the major reasons for glioblastoma treatment failure is the complexity of its tumor microenvironment (TME). The tumor is composed not only of malignant cells but also of infiltrating myeloid cells, cancer-associated fibroblasts (CAFs), and mesenchymal stem cells (MSCs). These supporting cells secrete various factors, including matrix metalloproteases (MMPs), fatty acids, transforming growth factor-beta (TGF-β), and chemokines like CCL5, which promote tumor invasion, suppress immune responses, and reduce treatment effectiveness5. Additionally, the TME is characterized by hypoxia, acidosis, angiogenesis, and high levels of immunosuppression. These conditions limit drug delivery, promote resistance to radiation and chemotherapy, and impair the function of therapeutic immune cells. As a result, even promising strategies like CAR T cells and checkpoint inhibitors face significant obstacles in treating GBM effectively6.
AD is the most common cause of dementia and is marked by memory loss, amyloid plaques, tau tangles, and chronic neuroinflammation. Drugs such as acetylcholinesterase inhibitors and NMDA receptor antagonists offer only mild symptom relief 7, while new antibody-based therapies like lecanemab show only modest results in slowing cognitive decline8. Emerging research also shows that alterations in gut microbiota composition may contribute to Alzheimer’s disease progression. A study by Cattaneo et al. (2017) found that AD patients had increased levels of pro-inflammatory bacteria such as Escherichia/Shigella and decreased levels of anti-inflammatory species like Eubacterium rectale when compared to cognitively healthy individuals9. The study also reported a significant reduction in overall microbial diversity. This microbial imbalance is associated with elevated systemic inflammation and may influence amyloid plaque accumulation and cognitive decline. More recent findings by Seo and Holtzman (2024) support the idea that targeting gut microbiota to restore anti-inflammatory populations and enhance microbial diversity could offer a promising therapeutic strategy10.
PD affects around 10 million people and is caused by the loss of dopamine-producing neurons in the substantia nigra. Treatments like levodopa, MAO-B inhibitors, and deep brain stimulation manage symptoms but do not prevent disease progression11. Diet also plays a key role in shaping the gut microbiome in Parkinson’s disease. A recent study by Kwon et al. (2024) found that high sugar intake was associated with increased neuroinflammation and a higher abundance of pro-inflammatory gut bacteria, particularly Klebsiella species12. These microbial changes were linked to worsened motor symptoms and a more rapid progression of disease in mouse models. The findings suggest that dietary factors can influence the gut-brain axis by modifying microbial composition and inflammatory status. Reducing dietary sugar and supporting a more diverse microbiome may therefore represent a modifiable intervention point in Parkinson’s disease management.
MS is a chronic autoimmune condition marked by demyelination and inflammation in the CNS.
Therapies like interferons, glatiramer acetate, and ocrelizumab reduce relapse rates but do not reverse damage or cure the disease13. While most MS treatments aim to reduce immune system activity, they do not cure the disease, in part because they fail to address deeper neurological damage. One reason is the failure of remyelination at lesion sites, which is caused by a lack of oligodendrocyte precursor cells capable of regenerating myelin sheaths14. Even when inflammation is suppressed, damaged neurons may not recover due to this regenerative failure. Another factor is molecular mimicry, where viral proteins resemble components of the myelin sheath. This confusion can lead to autoimmune T cell responses that sustain CNS damage. The gut microbiome may also contribute to disease onset through immune priming. Setiadi et al. (2017) showed that microbiota from MS mouse models, when transferred into wild-type mice, increased susceptibility to autoimmune demyelination15. These findings suggest that immune suppression alone is not enough and that regenerative failure and microbial factors must also be addressed in future MS therapies.
Disease | Standard Treatments | Limitations | Quantitative Outcomes |
Glioblastoma | Surgery, radiotherapy, temozolomide | High recurrence, poor BBB drug delivery,resistance | Median overall survival is~15 months; 2-year survival rate: 27% |
Alzheimer’s | Cholinesterase inhibitors, memantine | Modest symptom relief, does notslow disease progression | MMSE score improves by~2–3 points over 6 months inmild-to-moderate AD |
Multiple Sclerosis | Interferons, monoclonal antibodies (e.g., natalizumab) | Risk of infections, incomplete remission | Reduces relapse rates by~30%–68% depending on drug used |
Parkinson’s | Levodopa, dopamine agonists | Wearing off, dyskinesia, no cure | Levodopa improves motor scores (UPDRS-III) by~30–40% in early PD |
Research increasingly points to the gut-brain axis (GBA) as a factor that connects these conditions. The GBA links the gastrointestinal tract and central nervous system through immune, neural, and hormonal pathways. Microbial imbalance in the gut, known as dysbiosis, can trigger inflammation, affect the blood-brain barrier, and change how the immune system responds. In GBM, mouse studies show that manipulating the microbiome can improve responses to checkpoint inhibitors16. In AD, inflammation from the gut can worsen amyloid and tau buildup17. PD patients show early gut involvement and distinct microbial patterns, possibly connected to alpha-synuclein spreading through the vagus nerve18. MS models also reveal that microbiome changes influence immune activity and disease progression19. Together, this evidence supports the idea that targeting the GBA could help across multiple brain diseases. Treatments like probiotics, prebiotics, fecal microbiota transplantation, and dietary adjustments are being explored as ways to restore microbial balance and reduce inflammation. These approaches may work alongside existing therapies to address disease more holistically, beginning not just in the brain but in the body’s wider systems.
Shared Mechanisms Linking Gut and Brain in Disease
Even though glioblastoma, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis appear very different in their symptoms, they often share core biological pathways. One of the most important of these is dysregulation of the gut-brain axis. When the gut microbiome becomes unbalanced, it can trigger a chain of events that impact brain function, increase inflammation, and even influence how severe a disease becomes. Across these conditions, key shared mechanisms include chronic inflammation, breakdown of the blood-brain barrier, changes in microbial signaling, vagus nerve communication, and immune system disruptions.
The enteric nervous system (ENS), sometimes called the “second brain,” is a dense network of around 500 million neurons embedded in the lining of the gastrointestinal tract. It can operate independently of the central nervous system and plays a major role in regulating digestion, gut motility, and local immune responses. Through the vagus nerve and spinal pathways, the ENS communicates directly with the brain and responds to signals from gut microbes. These microbes produce neurotransmitters such as serotonin, dopamine, and GABA, which can influence brain function and mood. When gut microbial diversity is reduced, these signaling pathways may become disrupted. In the brain, this imbalance can activate resident immune cells such as microglia and astrocytes. These cells respond to inflammatory signals originating from the gut, leading to sustained neuroinflammation and the release of neurotoxic molecules that contribute to disease progression in Alzheimer’s, Parkinson’s, and multiple sclerosis20.
Chronic inflammation is a major factor that links gut imbalance to brain-related damage. An unhealthy gut microbiome can lead to high levels of inflammatory molecules like interleukin-6 and tumor necrosis factor-alpha. These cytokines not only drive inflammation in the brain but also contribute directly to tumor growth in glioblastoma and nerve cell loss in Alzheimer’s and multiple sclerosis16. Because these molecules can cross the blood-brain barrier, they connect immune system changes in the body to damage in the central nervous system. The immune system plays a central role in gut-brain axis dysfunction. Toll-like receptors (TLRs) on immune and epithelial cells detect microbial fragments such as lipopolysaccharide (LPS) and activate innate immune responses. When dysbiosis occurs, this signaling becomes excessive, driving chronic inflammation. A key consequence is an imbalance between regulatory T cells (Tregs), which maintain immune tolerance, and T helper 17 (Th17) cells, which promote inflammation. In disease states, Tregs decrease while Th17 cells increase, leading to the release of cytokines such as IL-6, TNF-α, and IL-1β, and a reduction in anti-inflammatory cytokines like IL-10. This shift creates a pro-inflammatory environment that affects both the gut and brain, worsening neurodegenerative and autoimmune conditions1.
Another important immune mechanism is molecular mimicry, where bacterial proteins resemble neural antigens. This resemblance confuses the immune system and may lead to autoimmune attacks on brain tissue. In multiple sclerosis, gut microbes have been shown to produce antigens that mimic myelin proteins, triggering damaging T cell responses. A study by Bjørnevik et al. (2022) found that gut microbiota from MS patients could induce spontaneous autoimmune inflammation when transferred into healthy mice, supporting the idea that gut-derived mimicry may help drive disease onset21.
Gut dysbiosis can also weaken the blood-brain barrier itself, making it easier for harmful signals and microbial fragments to reach the brain. This has been linked to cognitive decline in Alzheimer’s and a worsened tumor environment in glioblastoma17. Another shared mechanism involves short-chain fatty acids (SCFAs) like butyrate, acetate, and propionate. These molecules, which are produced by certain gut bacteria, help support brain health by regulating immune responses and maintaining the blood-brain barrier. When SCFA levels drop, as seen in Parkinson’s disease and multiple sclerosis, symptoms often become more severe19. The vagus nerve acts as a direct line of communication between the gut and brain. In Parkinson’s disease, studies suggest that misfolded alpha-synuclein may travel along this nerve from the gut to the brain, contributing to the spread of the disease18. The immune system is also strongly shaped by the gut microbiota. When the microbiome is unbalanced, it can lead to an increase in inflammatory T cells and a drop in protective regulatory T cells. This kind of immune shift is often seen in multiple sclerosis and other conditions where inflammation worsens over time19.
Short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate also influence gut-brain signaling by binding to G-protein coupled receptors on immune and epithelial cells. These receptors include free fatty acid receptor 2 (FFAR2 or GPR43), FFAR3, and hydroxycarboxylic acid receptor 2 (HCA2), which are highly expressed on regulatory T cells (Tregs) in the intestinal mucosa. When SCFAs engage these receptors, they promote anti-inflammatory responses and help maintain intestinal immune balance. However, in dysbiosis, SCFA levels fall and receptor signaling is reduced. This disrupts Treg function and contributes to a pro-inflammatory environment that can impair blood-brain barrier integrity and promote neuroinflammation. This receptor-mediated pathway helps explain how microbial metabolites like SCFAs can directly influence brain health through immune modulation22.
Gut-brain interventions may also affect synaptic plasticity, which is crucial for learning, memory, and recovery from neurological damage. Certain microbial metabolites, especially short-chain fatty acids like butyrate, have been shown to increase the expression of brain-derived neurotrophic factor (BDNF), a protein that supports synapse formation and neural survival. Probiotic treatments and high-fiber diets that restore gut microbiota diversity have also been linked to improved synaptic signaling and dendritic spine density in animal models of neurodegeneration. These effects suggest that targeting the gut may help not only reduce inflammation but also directly support cognitive resilience23.
All of these overlapping pathways show that gut health has a major influence on brain diseases. They help explain why patients with different conditions may still experience similar treatment challenges and why therapies that support gut balance could have wide-reaching benefits. By better understanding these shared disruptions, researchers may find new ways to treat diseases earlier and more effectively, not just by targeting the brain, but by focusing on the body as a whole.
Evaluating Current Treatments and Unmet Needs
Current treatments for glioblastoma, Alzheimer’s, Parkinson’s, and multiple sclerosis mainly focus on managing symptoms and slowing disease progression. While they offer some benefits, these therapies often fail to address deeper biological issues like chronic inflammation, immune dysfunction, and gut-brain axis involvement. In glioblastoma, surgery, radiation, and temozolomide are standard but rarely prevent relapse, and median survival remains around 15 months3. Newer treatments like checkpoint inhibitors and CAR T cells have not shown major success, partly because of immune suppression in the tumor environment and poor drug access to the brain4. In Alzheimer’s, drugs like donepezil and memantine provide short-term relief but do not change disease progression7. Antibody therapies such as lecanemab show promise but are expensive, invasive, and offer only limited benefit8. These treatments do not directly target the inflammation and blood-brain barrier problems that may drive the disease.
For Parkinson’s, medications like levodopa help with motor symptoms early on, but long-term use causes side effects and does not stop neuron loss11. Deep brain stimulation helps some patients but is invasive and not widely accessible. Non-motor symptoms, especially those related to gut health, remain difficult to treat. MS therapies like interferons and ocrelizumab reduce relapses but do not cure or reverse damage and may suppress the immune system13. With gut microbes shown to influence immune function in MS, current treatments overlook this part of disease biology.
Across all four conditions, most therapies focus on the brain itself and leave out systemic factors like gut imbalance and immune shifts. As research into the gut-brain axis grows, new therapies may offer more holistic and earlier interventions that work with the body’s full systems instead of just treating symptoms after damage has occurred.
Innovative Therapeutic Approaches Targeting the Gut-Brain Axis
The gut-brain axis (GBA) is a two-way communication system that links the gastrointestinal tract to the central nervous system through neural, immune, and hormonal pathways. It helps maintain balance in the body and plays an important role in brain function. When this system is disrupted, it can lead to or worsen conditions like Alzheimer’s disease, Parkinson’s disease, and glioblastoma. New treatment strategies aim to restore this balance by targeting the gut microbiome to reduce inflammation, protect brain cells, and improve symptoms.
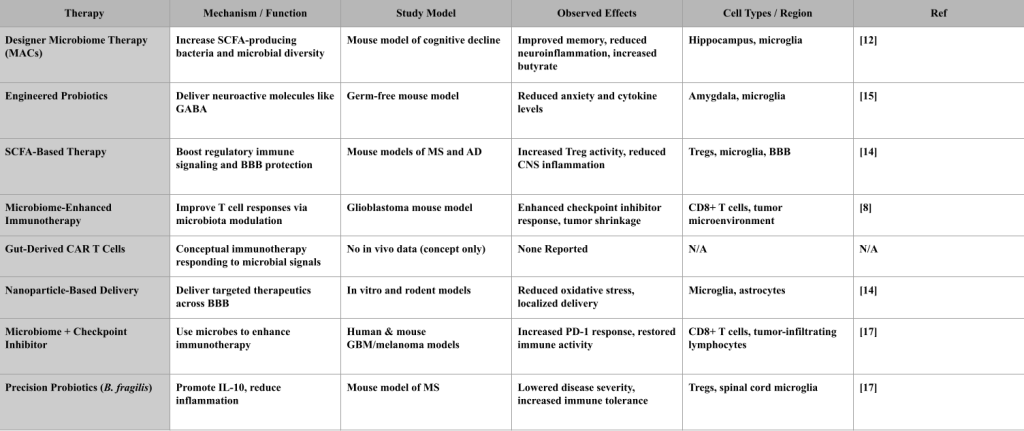
Designer Microbiome Therapies involve reshaping the gut microbiota by using special nutrients called microbiota-accessible carbohydrates (MACs). These increase the abundance of SCFA-producing bacteria, particularly butyrate-producing strains. In a mouse model of diet-induced cognitive decline, MAC supplementation raised butyrate levels, improved gut microbial diversity, and reduced brain inflammation. The study showed protective effects on hippocampal neurons and tight junction proteins in the blood-brain barrier24.
Engineered Probiotics are genetically modified bacteria designed to deliver neuroactive compounds. In a study using Lactobacillus reuteri engineered to produce GABA, germ-free mice exhibited reduced anxiety-like behaviors and lower brain levels of inflammatory cytokines. The effects were linked to changes in microglial activation and increased GABA levels in the Amygdala25. SCFA-Based Therapies aim to boost levels of butyrate, acetate, and propionate, which are produced by fiber fermentation. In mouse models of Alzheimer’s disease and multiple sclerosis, SCFA supplementation increased Treg cell activity, reduced microglial activation, and helped preserve blood-brain barrier integrity. The treatment also improved learning and memory in behavioral assays26.
Microbiome-Enhanced Immunotherapies improve the effectiveness of immune-based treatments by first restoring microbial balance. In a glioblastoma mouse model, gut microbiota modulation using antibiotics and fecal microbiota transplantation enhanced the response to PD-1 checkpoint inhibitors. The results showed increased infiltration of CD8+ T cells into the tumor and reduced tumor volume25.
Gut-Derived CAR T Cells are a conceptual strategy exploring whether immune cells could be engineered to respond to microbial signals. While this idea reflects growing interest in microbiome-influenced immunotherapy, it remains hypothetical. There are no preclinical or clinical studies demonstrating that CAR T cells can respond to gut-derived cues, cross the blood-brain barrier, or specifically target brain disease. Major barriers include unclear antigen targets, lack of delivery mechanisms, and potential autoimmune risks. This approach is still in its theoretical stage and requires extensive validation27.
Nanoparticle-Based Delivery Systems are designed to cross the blood-brain barrier and deliver therapeutic molecules directly to affected brain regions. In both in vitro BBB models and rodent studies, nanoparticles coated with targeting ligands successfully delivered anti-inflammatory compounds to microglia and astrocytes in the cortex. The treatment reduced oxidative stress and localized inflammation26.
Checkpoint Inhibitor–Microbiome Synergy is based on findings that certain gut bacteria improve cancer immunotherapy outcomes. In both preclinical and human studies, the presence of Akkermansia muciniphila was associated with better responses to PD-1 blockade in glioblastoma and melanoma. Fecal microbiota transplant from responder patients into germ-free mice conferred similar sensitivity to checkpoint inhibitors28.
Precision Anti-Inflammatory Probiotics like Bacteroides fragilis are designed to produce specific molecules such as polysaccharide A (PSA), which increases IL-10 production and modulates regulatory immune responses. While it reduces inflammation in the CNS, it does not reverse disease pathology. In a mouse model of multiple sclerosis, oral administration of B.
fragilis reduced disease severity, lowered microglial activation in the spinal cord, and shifted the Treg/Th17 balance toward immune tolerance28.
Three-Tiered Microbiome Therapy Model
To organize the growing range of gut-brain therapeutic strategies, I propose a conceptual model that groups them into three biologically distinct but interconnected categories: Microbiome Restoration, Immunomodulation, and Neuroprotection. These tiers reflect the key checkpoints where dysfunction in the gut-brain axis contributes to disease. By separating interventions based on the main biological system they influence, the model helps clarify how each therapy works and where it fits into broader treatment planning.
Tier 1: Microbiome Restoration targets the root causes of gut-brain axis dysfunction. Many neurological diseases begin with an imbalance in the gut microbiome, known as dysbiosis, which disrupts the production of protective metabolites like short-chain fatty acids (SCFAs) and allows pro-inflammatory bacteria to thrive22. Therapies in this category aim to restore microbial balance and improve the gut barrier. Examples include fecal microbiota transplantation (FMT), which introduces beneficial microbes from healthy donors, as well as synbiotics and engineered probiotics that promote the growth of SCFA-producing strains. These interventions support epithelial barrier integrity and help prevent harmful immune activation.
Tier 2: Immunomodulation addresses the immune system responses that link gut health to brain inflammation. Dysbiosis can lead to an increase in pro-inflammatory cells, such as Th17, and a reduction in regulatory T cells (Tregs), tipping the immune system toward a state of chronic activation. Therapies in this tier work to restore balance by reducing the release of inflammatory cytokines and boosting regulatory pathways. For example, SCFA supplementation promotes Treg differentiation and helps quiet overactive immune responses29. Other strategies include probiotics like Bacteroides fragilis, which produces polysaccharide A to encourage immune tolerance, and the use of checkpoint inhibitors paired with microbiota modulation to enhance anti-tumor immunity30. These approaches are especially relevant in conditions like multiple sclerosis and glioblastoma, where immune imbalance plays a central role.
Tier 3: Neuroprotection focuses on protecting the brain itself. By the time the gut and immune systems are disrupted, there may already be damage to the central nervous system, including blood-brain barrier (BBB) breakdown and glial cell overactivation. This tier includes therapies that reduce neuroinflammation and support the function of neurons and glia. Examples include nanoparticle-based delivery systems that carry anti-inflammatory compounds across the BBB, as well as SCFA-producing microbes that help stabilize endothelial junctions and suppress microglial activation31. The goal is to limit further damage and preserve cognitive and neurological function. What makes this model biologically meaningful is the way it reflects the natural progression of disease. Most conditions involving the gut-brain axis begin with microbiome disturbance, followed by immune changes, and finally lead to neuronal injury. Although the tiers are presented in this order, they are not separate or sequential. They influence one another in feedback loops. For instance, restoring a healthy microbiome can reduce immune activation, and reducing inflammation can help protect the blood-brain barrier. Many therapies act across more than one tier, and their benefits can compound over time. Different diseases may require a different focus within the model. In Alzheimer’s disease, gut microbial composition may be the first target, while in Parkinson’s disease, the emphasis may shift to neuroprotection. Multiple sclerosis often requires immunomodulation to address its autoimmune nature, while glioblastoma may demand a combination of immune and barrier-based strategies due to the tumor’s highly immunosuppressive environment. The model is flexible and can be adapted based on the biology of each disease and the needs of the individual patient.
However, this framework has limitations. Some therapies do not fit neatly into a single tier, and our current understanding of how gut-brain therapies work in humans is still developing. There are also questions about which biomarkers can best guide therapy selection and how to match treatments to specific subtypes of disease. As more is learned about the gut-brain connection, this model may evolve to include additional categories or more refined classifications. Even with these caveats, the three-tiered approach provides a useful way to think about gut-brain therapies in a structured and personalized way. It emphasizes that no single treatment is likely to work for every patient, but that by addressing the gut, the immune system, and the brain together, more effective strategies may emerge.

Other Emerging or Adjunct Gut-Brain Interventions
In addition to the therapeutic strategies detailed above, several emerging or complementary interventions are gaining attention for their influence on gut-brain axis health.
Dietary interventions, particularly high-fiber, plant-rich, and low-sugar diets, have been shown to increase the abundance of beneficial SCFA-producing bacteria and reduce gut permeability. Diet modifications are especially important in neurodegenerative conditions like Parkinson’s disease, where sugar intake has been linked to gut dysbiosis and neuroinflammation12. In both human and animal studies, Mediterranean-style diets are associated with improved microbiota diversity and lower levels of systemic inflammation32.
Exercise is another modifiable factor that influences both gut microbial composition and neuroinflammation. Regular aerobic activity has been shown to promote microbial diversity, increase SCFA levels, and improve cognitive function in mouse models of Alzheimer’s disease and human aging populations33.
Vagus nerve stimulation (VNS), traditionally used in treatment-resistant epilepsy and depression, is now being investigated as a means of modulating gut-brain communication. Preclinical studies suggest that VNS can reduce brain inflammation, improve autonomic balance, and indirectly shape gut microbiota profiles through neuroimmune pathways34.
Finally, bioengineered systems such as gut-brain-on-a-chip and organoid co-culture models are being developed to simulate the bidirectional communication between intestinal and neural tissues. These technologies offer a high-throughput way to test therapeutic compounds and investigate mechanisms of gut-brain dysfunction in a controlled, physiologically relevant environment35.
Broader Applications of Gut-Brain Axis Research
While much of this review focuses on glioblastoma and neurodegenerative diseases, the gut-brain axis (GBA) has shown potential across a wide range of conditions. In mental health, gut bacteria influence neurotransmitters like serotonin and GABA. Studies show that probiotics can improve mood and anxiety, and a clinical trial by Kazemi et al. (2019) found reduced depression scores in patients taking probiotic supplements36. The GBA is also involved in autoimmune diseases like multiple sclerosis and type 1 diabetes, where imbalanced gut microbes contribute to chronic inflammation and immune overactivation19.
The GBA also connects to metabolic disorders such as obesity and type 2 diabetes. Specific gut microbes and their metabolites directly influence host metabolism. For example, short-chain fatty acids (SCFAs) such as butyrate and propionate, produced by Akkermansia muciniphila and Faecalibacterium prausnitzii, interact with free fatty acid receptors (FFAR2/3) on enteroendocrine cells to modulate GLP-1 and PYY secretion, which suppress appetite and enhance insulin sensitivity37. Additionally, Akkermansia muciniphila has been associated with improved glucose homeostasis and reduced adiposity in both animal models and human studies38. In oncology, the GBA is being studied in other brain cancers like medulloblastoma and metastatic brain tumors, where the gut may shape immune responses and affect how tumors react to treatment. Favorable microbiome profiles have also been linked to better outcomes with checkpoint inhibitors in cancers like melanoma and lung cancer39.
These findings suggest the GBA is not only central to brain health but may also influence broader disease processes throughout the body. As microbiome-based treatments grow, they may offer personalized strategies for psychiatric, autoimmune, and cancer-related conditions.
Conclusion
The gut-brain axis is becoming an important framework for understanding brain-based diseases that were once viewed in isolation. Conditions like glioblastoma, Alzheimer’s, Parkinson’s, and multiple sclerosis share common biological disruptions such as chronic inflammation, immune imbalance, and microbiome changes. These patterns suggest that the gut may play a more central role in both disease progression and treatment than previously thought. Traditional treatments often focus only on the brain, but newer strategies that target the gut-brain axis can work at earlier stages and address root causes. Approaches like engineered probiotics, short-chain fatty acid supplements, microbiome-based immunotherapies, and gut-directed CAR T cells show promise in reducing neuroinflammation, repairing the blood-brain barrier, and supporting immune health. By treating the body and brain as a connected system, these interventions may help improve biological markers in preclinical models of neurological and oncological diseases, though human evidence remains limited. However, evidence from human trials remains limited, and more research is needed to determine their clinical effectiveness.
However, the success of these therapies will likely depend on precision medicine strategies that match the right treatment to the right patient. Stratifying individuals based on microbiome composition, immune markers, and disease subtype will be essential for translating these findings into clinical benefit. As research continues, gut-brain therapies could become a key part of personalized medicine, offering new hope for patients facing diseases that are currently hard to treat.
Methods
This literature review was done by searching databases like PubMed, Google Scholar, and ScienceDirect for primary research and review papers about the gut-brain axis and its role in brain cancer and neurodegenerative diseases. Keywords like “gut-brain axis,” “glioblastoma,” “Parkinson’s disease,” “Alzheimer’s,” and “short-chain fatty acids” were used. Most papers were published in the last ten years, but a few older ones were included if they made important contributions. Papers were first screened by their titles and abstracts to see if they were relevant to the research question. Only English-language studies were included. More detailed reviews of introductions, subheadings, and results were done to decide if the papers had useful information about shared biological pathways and potential treatments. The focus was on papers that connected the GBA to chronic inflammation, immune dysregulation, and possible therapeutic strategies like engineered probiotics or SCFA-based therapies. This method helped identify recent and useful papers, but it may have missed deeper insights from studies that didn’t stand out at first glance.
References
- K.J. O’Riordan, M.R. Collins, S.A. Moloney, et al. The gut microbiota–immune–brain axis: Therapeutic implications. Cell Rep. Med. 6, 101982 (2025). [↩] [↩]
- C. Shi, Y. Zhang, D. Huang, et al. Dietary fiber modulates gut microbiota and improves cognition in obese mice. Nutr. Neurosci. 23, 434–443 (2020). [↩]
- C. H. van Dyck, R. S. Swanson, J. W. Aisen, et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023). [↩] [↩]
- J. Y. Szeto, S. J. Lewis. Current treatment options for Alzheimer’s disease and Parkinson’s disease dementia. Curr. Neuropharmacol. 14, 326–338 (2016). [↩] [↩]
- L. V. Kalia, A. E. Lang. Parkinson’s disease. Lancet. 386, 896–912 (2015). [↩]
- S. L. Hauser, D. L. Bar-Or, F. Comi, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017). [↩]
- J. S. Ostrom, L. M. Gittleman, G. Truitt, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15 Suppl 2, ii1–ii56 (2013). [↩] [↩]
- M. Lim, A. Xia, A. Bettegowda, C. G. Weller. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 15, 422–442 (2018). [↩] [↩]
- J. Berer, S. M. Mues, A. Koutrolos, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA. 114, 10719–10724 (2017). [↩]
- B. Mulak. A link between irritable bowel syndrome and Parkinson’s disease: Clinical implications. World J. Gastroenterol. 21, 10609–10616 (2015). [↩]
- S. Liu, J. Cheng, L. Lin, D. Zhang. Vagus nerve and the gut–brain axis: Physiology and clinical implications. J. Clin. Neurosci. 45, 6–10 (2017). [↩] [↩]
- Y. Chen, Q. Xu, W. Wang, et al. Gut microbiota metabolites in Alzheimer’s disease: Current understanding and future prospects. Front. Aging Neurosci. 13, 650517 (2021). [↩] [↩]
- M. Feustel, J. L. Saunderson, M. Boehm, B. L. Greten, K. K. Robson. Microbiome-driven enhancement of checkpoint inhibitor response in glioblastoma. Cell Rep. Med. 3, 100604 (2022). [↩] [↩]
- S. Chandra, T. Tiwari, A. Singh. Gut-brain axis dysfunction in Alzheimer’s disease and the potential therapeutic role of microbiota modulation. Mol. Neurobiol. 60, 1275–1294 (2023). [↩]
- T. Tanoue, R. Atarashi, K. Honda. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 21, 295–309 (2021). [↩]
- A. Kazemi, M. Noorbala, M. Azam, et al. Effect of probiotic and prebiotic supplementation on depression symptoms and inflammatory markers in patients with major depressive disorder: A double-blind, randomized, placebo-controlled trial. Nutr. Neurosci. 22, 598–604 (2019). [↩] [↩]
- L. Derosa, V. Routy, S. Fidelle, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 80, 55–63 (2021). [↩] [↩]
- X. Zhao, L. Zhang, Q. Li, et al. Gut microbiota composition modifies fecal metabolic profiles in children with obesity. Front. Microbiol. 9, 2084 (2018). [↩] [↩]
- A. Plovier, L. Everard, C. Druart, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017). [↩] [↩] [↩] [↩]
- J. Canfora, J. W. van der Beek, E. M. Blaak. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591 (2015). [↩]
- G. Agirman, C. Yu, E. Y. Hsiao. Signaling inflammation across the gut-brain axis. Science. 374, 1087–1092 (2021). [↩]
- K. J. O’Riordan, M. R. Collins, S. A. Moloney, et al. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 546, 111572 (2022). [↩] [↩]
- Y. P. Silva, A. Bernardi, R. L. Frozza. The role of short-chain fatty acids from gut microbiota in gut–brain communication. Front. Endocrinol. 11, 25 (2020). [↩]
- Bjørnevik, K., Cortese, M., Healy, B. C., et al. Gut microbiota from multiple sclerosis patients induces autoimmune encephalomyelitis in mice. Science. 377, 202–208 (2022). [↩]
- M. J. Son, K. Woolard, D. H. Nam, J. Lee, H. A. Fine. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 4, 440–452 (2016). [↩] [↩]
- R. Saleh, E. Elkord. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin. Cancer Biol. 65, 13–27 (2020). [↩] [↩]
- A. Cattaneo, N. Cattane, S. Galluzzi, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Brain Behav. Immun. 62, 112–118 (2017). [↩]
- D. O. Seo, D. M. Holtzman. Microbiota modulation as a therapeutic strategy in Alzheimer’s disease. Trends Neurosci. 47, 180–194 (2024). [↩] [↩]
- S. H. Kwon, B. J. Yoon, M. E. Han, et al. High sugar intake promotes gut dysbiosis and neuroinflammation in a mouse model of Parkinson’s disease. Cell Rep. 47, 108345 (2024). [↩]
- S. Mi, R. H. Miller, X. Lee, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 8, 745–751 (2007). [↩]
- A. F. Setiadi, K. M. Omari, W. J. Karpus. Gut microbiota from multiple sclerosis models drives autoimmune responses in naïve mice. J. Immunol. 198, 2102–2112 (2017). [↩]
- J. T. Loh, M. R. Hernandez, H. Y. Chua, et al. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Trends Mol. Med. 30, 88–103 (2024). [↩]
- Kwon, H. E., et al. Sugar-rich diet alters gut microbiota and worsens Parkinson’s pathology in mice. Neurobiol. Dis. 186, 106347 (2024).; T. S. Ghosh, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year randomized controlled trial. Gut. 69, 1218–1228 (2020). [↩]
- J. M. Allen, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 50, 747–757 (2018). [↩]
- J. J. Choi, et al. Exercise attenuates gut dysbiosis and neuroinflammation in Alzheimer’s disease mouse models. Brain Behav. Immun. 34, 21–30 (2013).; S. Breit, et al. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front. Psychiatry. 9, 44 (2018). [↩]
- S. Jalili-Firoozinezhad, et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 3, 520–531 (2019). [↩]
- W. Shin, H. J. Kim. Intestinal barrier dysfunction orchestrates the onset of inflammatory host–microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl. Acad. Sci. USA. 115, E10539–E10547 (2018). [↩]
- V. Fock, R. G. Parnova. Nanoparticles as carriers of therapeutic molecules for neurodegenerative diseases. Nanomedicine. 18, 1–18 (2023). [↩]
- E. Cuffaro, M. Ascone, C. Fiorani, et al. Probiotics, prebiotics and fecal microbiota transplantation as therapeutic strategies for neurodegenerative diseases. Int. J. Mol. Sci. 25, 2155 (2024). [↩]






