Abstract
Asthma is a prevalent respiratory condition affecting over 300 million people worldwide as of 2024, with substantial mortality rates each year. In 2019, there were 455,000 asthma-related deaths. Despite advances in patient care for asthma, there are still many asthma-related deaths occurring each year. Corticosteroids such as Flovent, Asmanex, and Qvar are the main form of medication to treat asthma, but users often experience adverse side effects like mouth and throat irritations, headaches, and chest pain. This makes it extremely hard for patients who are asked to take this medicine multiple times a day in order to treat their asthma. Recent advancements in asthma therapeutics have led to the development of new, non-steroid-based treatments with fewer side effects and improved efficiency over the long term, with one linking asthma to gut health. Emerging research introduces the concept of the “gut-lung axis”, which implies that the gut and lung microbiomes influence each other. This paper highlights the advancements in asthma therapeutics and provides a comprehensive review of the biology of asthma, deficiencies of current corticosteroid treatments, the mechanism of action of treatments, and new and emerging forms of treatment for asthma.
Keywords: Asthma; Action Plan; Inflammation; Gene Therapy; Gut-Lung Axis
Introduction
Asthma is a prevalent respiratory condition that affects millions worldwide. According to the National Library of Medicine, over 300 million people globally will have asthma in 20241. According to the National Center for Health Statistics, in the United States, 27 million people were affected by asthma, and 4.5 million of them were children in 20222. Asthma is one of the most common diseases in the United States, with 1 in 12 people affected by this condition3. In 2019, asthma caused 455,000 deaths worldwide and 3,542 in the United States4. 1,000 asthma-related deaths occur every day5. Many of these deaths occur in lower-income countries, as access to a proper diagnosis and treatment is limited6.
Asthma also has a notable economic impact. According to a CDC study conducted in 2018, medical expenses from asthma cost the U.S. economy over $80 billion annually7. Ongoing advancements in asthma treatments, include inhalers, biologics, and other medications designed to manage asthma symptoms and prevent asthma attacks.
Despite advances in patient care for asthma, there are still many asthma-related deaths occurring each year. Corticosteroids are the main form of medication to treat asthma, but these medications such as Flovent, Qvar, and Asmanex can have adverse side effects that make it extremely hard for patients to take medicine multiple times a day to treat their asthma. This paper will highlight the biology of asthma, deficiencies of current corticosteroid treatments, the mechanism of action of treatments, and new and emerging forms of treatment for asthma, including recent discoveries linking gut health and asthma.
Objective
This review aims to study advancements in asthma therapeutics across diverse populations with a focus on addressing limitations of conventional treatments. We highlight three areas: (1) biologics targeting inflammatory cytokines (2) gene editing directed at immune pathways underlying chronic inflammation (3) emerging role of the gut-lung axis in modulating asthma. By synthesizing research across these domains, this review seeks to clarify the evolving treatment landscape and identify promising directions for more targeted and accessible asthma management.
Methods
This paper is a review of research on asthma treatments. Literature was identified by searching PubMed and Google Scholar with terms like “asthma treatment,” “corticosteroids,” “bronchodilators,” “dupilumab,” “mepolizumab,” “benralizumab,” “reslizumab,” “omalizumab,” “tezepelumab,” “gene therapy,” “CRISPR,” “gut-lung axis,” and “short-chain fatty acids.” Papers published from 2000 to 2024 were considered, focusing on clinical trials, review articles, and guidelines from major health organizations like GINA, WHO, and the CDC. Both kids and adults with asthma were included, especially those with moderate to severe asthma. Case reports or studies that were not about asthma treatments were excluded. Figures 1–4 were made using BioRender.com and are original.
Biology of Asthma
Asthmatic events can be triggered by a variety of factors, including allergens and respiratory infections. Common allergens that provoke asthma symptoms include pollen, dust mites, pet dander, and mold. Environmental irritants such as air pollution, cigarette smoke, and chemical fumes can also aggravate asthma. Additionally, respiratory infections, particularly infections like COVID-19, the common cold, or the flu, are significant triggers for asthma. Physical activity or stress can also induce asthma symptoms in some individuals. Asthma is typically accompanied with symptoms such as coughing, shortness of breath, wheezing, and chest pain. These symptoms are caused by the lining of the airways becoming swollen or inflamed, due to irritation from an allergen.
Genetic factors play a key role in determining who has asthma, and how severe it is. Certain genes can make people more likely to develop asthma by affecting the immune system and how their airways respond to inflammation. Some of the key genes involved are ORMDL3, ADAM33, HLA-DQ (A1/B1), and Filaggrin8. All the genes listed are involved in either inflammation, immunity, or lung function. For brevity, the two established genes, ADAM33 and ORMDL3 will be briefly discussed.
The ADAM33 gene contributes to asthma by causing changes in the structure and function of the airways. It affects the growth of fibroblasts, smooth muscles, and the deposition of matrix proteins, leading to thickening of the airway walls and making it harder to breathe. Some forms of ADAM33 can also cause abnormal blood vessel growth in the airways9.
The ORMDL3 gene, on the other hand, primarily influences immune system regulation and inflammation. It helps control sphingolipid metabolism, which is important for cell signaling. Disruption in this process can lead to increased inflammation in the airways. ORMDL3 is also involved in the unfolded protein response (UPR) within cells, which, when dysregulated, can also contribute to inflammation in the lungs. Elevated levels of ORMDL3 have been linked to increased production of pro-inflammatory cytokines, making the airways more sensitive to asthma symptoms10..
Mutations or polymorphisms occurring in any of these genes hinder their normal function, leading to an unbalanced immune system, decreased lung function, or airway hyperresponsiveness. These genes either contribute to early-onset asthma or cause susceptible adults to develop asthma later in life due to environmental factors such as smoking or pollen8.

On a molecular level, an asthma attack involves a series of reactions. When someone with asthma inhales an allergen, it is recognized by antigen-presenting cells in the airways (Figure 1) (15). These cells present antigens to Th2 (T-helper type 2), which release Th2 cytokines, specifically IL-5, IL-4, and IL-1315. IL-4 and IL-13 trigger B cells, which produce IgE (Immunoglobulin) and bind to the FcεRI of mast cells. When the same allergen is encountered, it binds to IgE, causing mast cells to release mediators such as leukotrienes or histamine15. IL-5 on the other hand, enables eosinophil (a type of white blood cell) production and maturation, and directs the eosinophils from the bloodstream into the lungs. Once in the lungs, eosinophils, along with other immune cells like mast cells, release substances such as histamine and leukotrienes that cause bronchospasm, the inflammation and swelling of the airway walls11.
Current Asthma Action Plans and Limitations
Asthma attacks can be life-threatening and typically require immediate intervention. An asthma action plan typically includes both bronchodilators and corticosteroids because they address different aspects of the disease and have complementary effects to manage symptoms and prevent asthma attacks. Corticosteroids help prevent inflammation and long-term damage, while bronchodilators provide immediate relief during asthma symptoms or attacks, ensuring optimal asthma control.
Bronchodilators such as albuterol or levalbuterol are the primary medications used for quick relief during an asthma attack. These bronchodilators work by targeting the beta-2 receptors on the muscle cells of the airways, causing these muscles to relax and subsequently dilate, making it easier to breathe. Bronchodilators are typically administered through inhalers or nebulizers, and provide relief of symptoms within minutes12. Their side effects are generally mild and dose-related, heart palpitations, tremor, or nervousness.
Corticosteroids, including inhaled corticosteroids (ICS) such as Flovent, QVAR, and Asmanex, act locally to reduce inflammation in the airways which plays a key role in asthma by causing mucus production and airway constriction. Corticosteroids also provide long-term control for asthma by reducing airway inflammation and hyperresponsiveness, which helps prevent and mitigate asthma attacks. While Corticosteroids are effective in asthma management, their side-effects are dose-related and can include headaches, mouth and throat irritation, chest pain, hives, and in rare cases, high blood pressure or vomiting13. Long-term use in children can also have adverse impact on growth. Corticosteroids primarily suppress inflammation and immune responses but do nothing to address the underlying specific mechanisms that trigger asthma. These confluence of factors highlight the need for alternative treatments that are more targeted in managing asthma.
Bronchodilators, such as albuterol, do not address underlying airway inflammation, and frequent use has been associated with increased risk of asthma-related hospitalizations13. Recent evidence supports the use of anti-inflammatory reliever therapy that combines rapid bronchodilation with anti-inflammatory action. Formoterol is a long-acting beta-2 agonist (LABA) that, when combined with inhaled corticosteroids, provides both immediate relief and suppression of airway inflammation. Studies have shown that formoterol significantly reduces the risk of severe exacerbations compared to bronchodilators and reduces overall corticosteroid exposure by allowing targeted use14. By addressing both bronchoconstriction and inflammation at the same time, anti-inflammatory reliever therapy represents a paradigm shift in asthma management.
Effective asthma care also relies on non-pharmacologic care. Personalized asthma action plans help patients recognize symptoms and adjust therapeutic response accordingly. Proper inhaler technique, often aided by spacers, maximizes drug delivery and efficacy. Reducing exposure to allergens, smoke, and environmental pollutants, can mitigate trigger-based flare ups. Routine vaccinations can reduce respiratory infection–related flare ups.
Recent Biological Therapies

Dupilumab is a monoclonal antibody that inhibits both IL-4 and IL-13. It works by binding to the IL-4 receptor alpha (IL-4Rα), which is part of the receptor network for both IL-4 and IL-13. By preventing these cytokines from interacting with their receptors, Dupilumab inhibits them from triggering other cells that lead to inflammation and bronchospasm (Figure 2)15. Dupilumab reduced flare ups and improved lung function in the LIBERTY ASTHMA QUEST trials16; however, most participants had type 2 asthma, limiting generalization. Dupilumab should be given as follows:
- Adults: Given by injection under the skin once every two weeks for indications of moderate-to-severe asthma with an eosinophilic phenotype or high Th-2 driven inflammation.
- Children: Given by injection under the skin once every two weeks for children older than 12, and once every fours week in children 6 months to 11 years old17. Indications of moderate-to-severe Type-II asthma not controlled by standard therapy.
There are also currently three FDA approved IL-5 blockers on the market for asthma. They are Mepolizumab, Benralizumab, and Reslizumab18. Mepolizumab is specifically used to prevent common asthma symptoms, such as wheezing, difficulty breathing, tightening of the chest, and coughing. This treatment is sold under the name Nucala, owned by the biopharma company GlaxoSmithKline. Mepolizumab lowered flare up rates and modestly improved lung function in the DREAM trials19. Benralizumab is also used to prevent asthma symptoms in those whose symptoms cannot be controlled by steroid medication. This medication is known as Fasenra, and is sold through AstraZeneca Pharmaceuticals. Benralizumab showed flare up reduction and steroid-sparing in the SIROCCO trials20, but response was strongest in eosinophilic phenotypes. Reslizumab is marketed under the name Cinqair, and was developed by Teva Pharmaceuticals. Reslizumab reduced flare ups and improved lung function in phase 3 trials21, though sample sizes were smaller and benefits were again confined to patients with elevated eosinophils. These IL-5 blockers work by binding to IL-5, which prevents it from interacting with its IL-5 receptor on the surface of eosinophils. By blocking IL-5, these treatments reduced the blood eosinophils count, leading to decreased inflammation and prevented asthma attacks. While these treatments are well accepted, they can cause side effects such as headaches, injection site reactions, upper respiratory tract infections, and in less frequent cases hypersensitivity and fatigue22. As such the treatments should be used with caution in certain patients. IL-5 blockers should be used with following guidance:
- Adults: Mepolizumab, Benralizumab, and Reslizumab are indicated for severe eosinophilic asthma that remains uncontrolled on inhaled corticosteroids. Mepolizumab is administered through injection once every four weeks. The first three doses of Benralizumab are given every four weeks, and then one dose every eight weeks. Reslizumab is given once every four weeks through an IV infusion. However, unlike the other two IL-5 blockers, Reslizumab is used specifically as an add-on treatment for severe eosinophilic asthma and is to be taken alongside oral steroid medications.
- Children: Mepolizumab is approved for children six years or older with similar criteria as adults. Benralizumab is approved for children 12 years or older with similar criteria as adults. Reslizumab is not currently approved for children.
Additional Biological Therapies
Beyond cytokine-targeted biologics, several other biological strategies help with asthma care.
- Omalizumab, an anti-IgE monoclonal antibody, is approved for moderate-to-severe allergic asthma uncontrolled by inhaled corticosteroids. Omalizumab reduces mast cell activation, thereby reducing allergic inflammation23.
- Tezepelumab, an anti-TSLP biologic, has demonstrated efficacy across a broad spectrum of asthma phenotypes by targeting an upstream epithelial cytokine that drives Type 2 inflammation24. Clinical trials have demonstrated that TSLP blockade significantly reduces asthma across eosinophilic and non-eosinophilic phenotypes. The NAVIGATOR phase-3 trials confirmed these findings in a larger, diverse population.25. These studies illustrate the strong potential of anti-TLSP therapy to provide broad protection against asthma.
Collectively, these therapies broaden the treatment landscape and provide tailored options for patients who do not respond adequately to conventional therapies.
Biological Therapies Comparison: Clinical Use and Outcomes
| Therapy | Target | Phenotype / Biomarkers | Main Indications | Delivery | Outcomes | Trade-offs |
|---|---|---|---|---|---|---|
| Dupilumab | IL4 / IL-13 | Type 2 inflammation | Moderate-to-severe asthma | SC injection; 2 weeks | Reduce flare ups, oral steroid sparing, | Broad coverage of Th2 asthma; injection-site reactions |
| Mepolizumab | IL-5 | Blood eosinophils ≥150/µL | Severe eosinophilic asthma | SC injection; 4 weeks | Reduce flare ups, oral steroid sparing | Specialist access required |
| Benralizumab | IL-5 | Blood eosinophils ≥300/µL | Severe eosinophilic asthma | SC injection; 4 weeks ×3, then 8 weeks | Reduce flare ups, steroid-sparing | Injection-site reactions |
| Reslizumab | IL-5 | Blood eosinophils ≥400/µL | Severe eosinophilic asthma, add-on therapy | IV infusion; 4 weeks | Reduce flare ups | IV route may be less convenient |
| Omalizumab | IgE | Elevated IgE | Moderate-to-severe allergic asthma | SC injection; 2–4 weeks | Reduce flare ups, steroid-sparing | Requires IgE testing |
| Tezepelumab | TSLP | Any phenotype | Severe asthma uncontrolled on standard therapy | SC injection; 4 weeks | Reduce flare ups, improved lung function | Relatively new; high cost |
While biological therapies are highly effective for selected asthma phenotypes they remain cost-prohibitive for widespread use. They are generally available through specialist care, often requiring prior authorization and confirmation of type 2 asthma. Estimated annual costs ranges from $25,000 to $45,00026. High prices limit treatment to patients with severe, uncontrolled asthma and amplify socioeconomic disparities. Access is extremely limited in low to middle income countries due to cost and infrastructure gaps. Addressing pricing and access strategies is essential to drive meaningful global health impact.
New Exploratory Gene Therapies
Gene therapy is a cutting-edge but exploratory approach to treat asthma by targeting specific genes involved with the disease. One of the most promising tools for gene therapy is the CRISPR-Cas9 system, which allows for precise gene editing. The CRISPR-Cas9 system has two main components: guide RNAs (gRNAs) and the Cas9 endonuclease. gRNAs are small pieces of genetic material designed to bind a specific gene within a cell. Once they identify the gene of interest, gRNAs direct Cas9 to the precise region of DNA that needs to be deleted or edited. When Cas9 cuts the gene specified by the gRNA, repair mechanisms are used to fix the break in the DNA. One method allows scientists to create gene knockouts that remove or inactivate a specific gene27.
Researchers at the Sean N. Parker Center for Allergy and Asthma Research at Stanford University are using CRISPR-Cas9 gene-editing technology to screen 800 kinases in the human body. This collaboration with Integrated DNA Technologies (IDT) aims to identify genes associated with asthma and allergies. By creating gene knockouts, scientists can study the role of specific genes in allergic reactions and asthma attacks, allowing them to better understand gene functions. This research may lead to discovering new drug targets and therapeutic treatments for asthma, potentially improving asthma symptoms27.
This technology can be extremely useful for asthma, as researchers can use CRISPR-Cas9 as a way to create gene knockouts in Th2-asthma related cells that were discussed earlier. Th2 cells are known to secrete inflammatory molecules (cytokines) that activate B cells to secrete IgE, a key substance involved in allergic diseases. When IgE binds to mast cells, an individual can develop sensitivity to that allergen. Upon exposure to allergens, mast cells immediately begin to secrete molecules such as histamine, which causes the common symptoms of asthma. Researchers have recently identified key genes associated with Th2 cells that may be involved in asthma. Through creating gene knockout cells through CRISPR-Cas9, it is possible to knockout disease-related Th2 cells, rendering them inactive and unable to produce substances that lead to asthma, such as IL-4, IL-5, and IL-1327.
While CRISPR is highly precise, it is not perfect and can lead to unintended gene editing that could cause harmful mutations. CRISPR may also trigger an immune response, potentially leading to inflammation or allergic reactions. Several key caveats must be considered:
- Asthma is polygenic with multiple susceptibility genes (e.g., ORMDL3, ADAM33, IL4, IL13) and environmental interactions. Targeting a single gene may have limited clinical benefit28.
- Effective editing requires delivery to specific immune cells (Th2, mast cells, eosinophils). The lung contains multiple cell types. In vivo targeting is technically difficult and off-target uptake could cause adverse effects29.
- Viral delivery vectors can efficiently deliver CRISPR components but may trigger immune responses. Nonviral delivery vectors are safer but often less efficient. Achieving uniform delivery throughout large, branching airway structures is difficult. Local delivery via aerosol may only reach nearby regions30.
- Gene-editing requires long-term follow-up (often up to 15 years) to monitor delayed adverse events. While somatic editing is permissible heritable editing remains off-limits.
- No published asthma trials exist and respiratory applications are mostly preclinical.
Gene-editing approaches for asthma remain experimental and are being explored primarily to help identify potential therapeutic targets but should not be interpreted as ready-to-use treatments. More research and safety assessments are required before clinical trials can ethically proceed.
Emerging Gut-Lung Axis
The gut-lung axis is an emerging area of research that explores how a person’s gut health can potentially impact lung health. This connection suggests that the state of our gut microbiome may influence conditions like asthma.
The gut microbiome includes fourteen core bacterial groups and 150 bacterial species, while the lung microbiome only has seven bacterial groups. One major difference between the two is that the gut microbiome has higher alpha diversity, meaning a wider variety of bacterial species are present in larger quantities. The dominant microbial phyla in the gut are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with Firmicutes and Bacteroidetes representing 90% of the gut microbiota. These include genera like Lactobacillus in Firmicutes and Bacteroides and Prevotella in Bacteroidetes31. In contrast, the lung microbiome has fewer dominant phyla, with Firmicutes and Bacteroidetes also present, but fewer genera overall, such as Prevotella, Porphyromonas, and Streptococcus32.
The diversity and abundance of species in the gut microbiome are important because they can influence immune function beyond the gut. The gut microbiome produces molecules like short-chain fatty acids (SCFAs), that can mobilize host immune cells that travel through the bloodstream to the lungs. These circulating cells may affect lung immunity and potentially change the composition of the lung microbiome.
This interaction is particularly relevant for asthma. The gut microbiome potentially affects asthma severity by influencing inflammation and immune responses. When the gut microbiome is out of balance, also known as dysbiosis, it can lead to lower levels of beneficial bacteria that produce short-chain fatty acids (SCFAs) like butyrate, which help reduce inflammation and maintain a healthy gut barrier. Although regular levels of SCFA protect against asthma symptoms to a certain extent, lower SCFA levels may worsen asthma by increasing inflammation and making the airway more susceptible to allergens, as the ability to activate Th2 cells is impaired33.

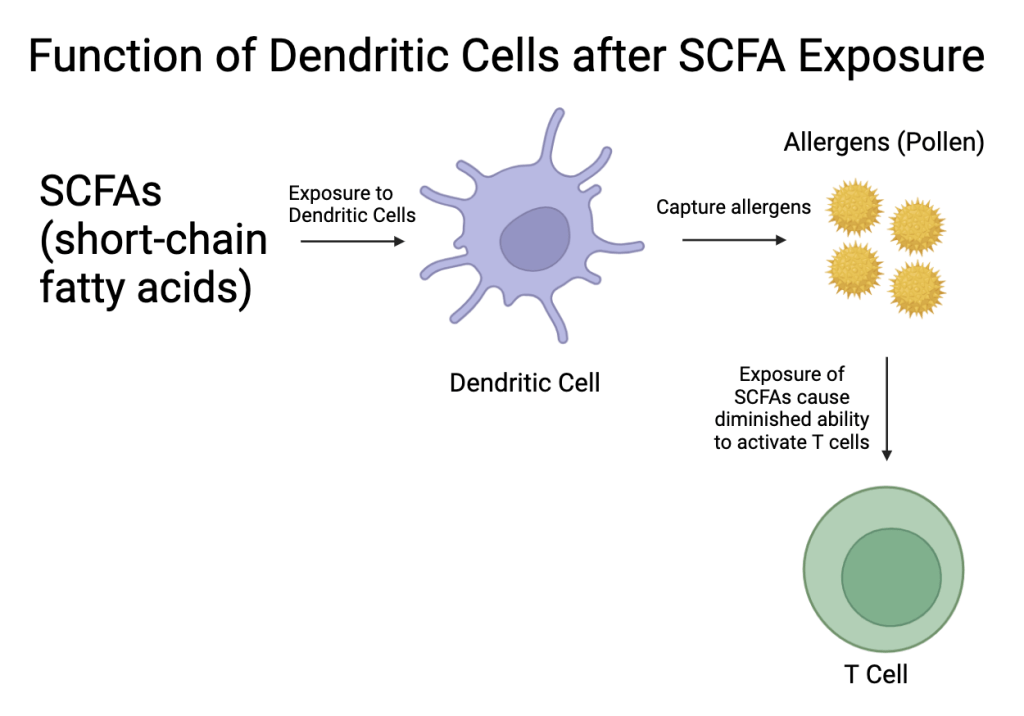
Short-chain fatty acids (SCFAs) from the gut can modulate dendritic cells and Th2 activation, reducing cytokine production and subsequent airway inflammation (Figure 3 & Figure 4)34.
Additionally, SCFAs modulate gene expression, specifically through the transcription factor FOXP3. By inhibiting histone deacetylation, SCFAs allow for the increased expression of FOXP3. FOXP3 is essential for the development and function of regulatory T cells (Tregs). Tregs help prevent the immune system from overreacting to harmless substances like allergens. This action is particularly important when considering asthma, where an overactive immune response can lead to inflammation and bronchospasms typical of asthma35.
The anti-inflammatory effects of SCFAs also reduce levels of circulating IgE, which is an antibody type associated with allergic responses. Studies have shown that dietary supplementation with SCFAs can decrease IgE levels, leading to a reduced risk of airway inflammation36. Likewise, the modulation of the gut microbiome by SCFAs has been linked to asthma outcomes. Lastly, SCFAs help increase the number of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), which are regulators in airway inflammation. PMN-MDSCs work together with Tregs to keep the immune system from overreacting and causing too much inflammation.
While these findings are promising, dietary interventions alone should not be considered a treatment for asthma at this time. Evidence from studies varies considerably and remains heterogeneous with confounding factors such as diet, environment and genetics.
- Gut microbiome composition and asthma: A study by Lancet Respiratory Medicine has shown that children with a gut microbiome capable of producing more SCFAs have decreased rates of asthma later in life37. However, children with a gut microbiome that produces less SCFAs have an increased risk of asthma development. SCFAs can reduce the number of IL-4 producing CD4+ T cells, which are crucial to the Th2 immune response that leads to asthma. This reduction in IL-4 production helps lessen the severity of asthma symptoms by reducing the body’s allergic responses36. Evidence from randomized clinical trials (RCTs) is limited – probiotic supplement trials have shown inconsistent effects on asthma occurrence.
- Short-chain fatty acids (SCFAs) and asthma risk: In a small cohort study, children with higher levels of butyrate and propionate in their feces at 1 year old have been found to have lower rates of atopic sensitization and are less likely to develop asthma later in childhood compared to those 3-6 years in age38. In a small animal study investigating myeloid-derived suppressor cells (MDSCs) and Treg cells, it was found that when mice treated with SCFA-containing drinking water, there was amelioration of allergic airway inflammation. The mice receiving the SCFA-containing water developed less severe asthma than the mice who didn’t receive it. The authors reported that this protective effect was due to the cooperation of PMN-MDSC and Treg induction39. To date, no large-scale randomized clinical trials (RCTs) have been conducted.
Ongoing targeted clinical trials are required to determine whether gut microbiome can influence asthma outcomes.
Comparison of Major Asthma Therapies
| Therapy | Action & Delivery | Main Indications | Side Effects | Cost / Access | Level of Evidence | Limitations / Risk of Bias |
|---|---|---|---|---|---|---|
| Inhaled Corticosteroids (ICS) | Suppress airway inflammation Inhaled per action plan | First-line for persistent asthma (all severities) | Headache, Mouth irritation, growth suppression (rare) | Widely available, generic, low cost | HighMultiple RCTs, long-term data | Adherence dependent; long-term growth suppression (rare) |
| Short-Acting B2-Agonists (SABA) | BronchodilationInhaled as needed | Rapid relief of symptoms; exercise-induced | Heart palpitation, Tremor, nervousness | Widely available, generic, very low cost | Moderate RCTs and decades of clinical use | Frequent reliance on observations |
| Anti-IL-4/IL-13 Biologics (Dupilumab) | Target cytokines (IL-4/13)SC injection | Moderate-to-severe asthma | Injection-site reactions | High cost | High (LIBERTY ASTHMA QUEST) Large phase 3 RCTs | Overrepresentation of oral CS-users; Long-term data limited |
| Anti-IL-5 (Mepolizumab,Benralizumab Reslizumab) | Target IL-5 cytokines to reduce type 2 inflammationSC Injection (reslizumab IV) | Severe eosinophilic asthma (reslizumab is add-on) | Injection-site reactions; IV less convenient | High cost, specialty access required | High (DREAM, SIROCCO; Multiple phase 3 RCTs | Mostly eosinophilic sample size; Small pediatric sample size |
| Anti-Ige (Omalizumab) | Target IgE to reduce type 2 inflammationSC Injection | Moderate-to-severe asthma | Injection-site reactions | High cost, requires IgE testing | HighMultiple phase 3 RCTs with long-term data | Selection bias toward allergic phenotype based on IgE levels |
| Anti-TSLP(Tezepelumab) | Target TSLP to reduce type 2 inflammationSC Injection | Severe asthma uncontrolled | Injection-site reactions | High cost, relatively new | High (Pathways, Navigator); Emerging large phase3 RCTs | Early clinical experience; limited long-term safety data. |
| Gene Editing | Gene therapy (CRISP for airway inflammation)Preclinical | Future potential for severe asthma | Unknown; off-target effects | Not available; limited to trials | LowAnimal studies/ preclinical in vivo | Off-target effects, delivery challenges, ethical/regulatory barriers, not clinically tested |
| Gut microbiome modulation | SFCAs, ProbioticsPreclinical or early phase clinical | Future potential for asthma control | Unknown; microbiome disruption | Not available; limited to trials | Animal studies, small human cohorts | Heterogeneous data, confounding factors (diet, environment) |
Conclusion
Asthma continues to be a major global health issue, affecting millions of people and causing significant health and economic challenges. Current treatments such as inhaled corticosteroids are the standard, and these therapies focus on reducing inflammation and quickly relieving bronchospasms. Inhaled corticosteroids, such as Flovent, Qvar, and Asmanex, are typically prescribed to control chronic asthma, while bronchodilators like albuterol provide rapid relief during acute attacks. However, many of these treatments come with side effects and sometimes may not even calm asthma symptoms, especially for those with severe forms of asthma.
Emerging treatments such as Dupilumab, Mepolizumab, Benralizumab, and Reslizumab, function by blocking IL-4, IL-5, and IL-13, pro-inflammatory cytokines that exacerbate asthma symptoms. Omalizumab (anti-IgE) has shown promise in patients with high IgE. Tezepelumab (anti-TSLP) has demonstrated broad efficacy in trials.
Also, gene-editing techniques like CRISPR-Cas9, show promise in providing effective care by knocking out disease-related Th2 cells, causing them to be unable to produce these pro-inflammatory cytokines. These advancements could help manage asthma better by addressing the underlying immune responses that lead to inflammation and breathing difficulties, and could potentially reduce the need for daily medications.
In addition to advancements in medication, the relationship between the gut microbiomes and lungs is a newer field in asthma research. The gut-lung axis suggests that maintaining a healthy gut microbiome, particularly through the production of short-chain fatty acids, can have protective effects against asthma by modulating the immune response and reducing inflammation. Ongoing research and advancement into the role of SCFAs, along with treatments such as gene therapy, could transform asthma care in the coming years, improving outcomes for patients and possibly reducing the global issue of asthma.
References
- Q. Wong, J. Lim, J. Ng, P. Malipeddi, Y. Lim, Y. Sio, F. Chew. An updated prevalence of asthma. National Institutes of Health. (2020). [↩]
- C. Pate, H. Zahran. The Status of Asthma in the United States. PubMed Central. (2024). [↩]
- A. Bowen, S. Nargundkar. Transformational Strategies to Improve the Clinical Trial and Drug Development Process. Open Journal of Safety Science and Technology. (2022). [↩]
- L. Yuan, J. Tao, J. Wang, W. She, Y. Zou, R. Li et al. Global, regional, national burden of asthma from 1990 to 2019. The Lancet Respiratory Medicine (2025). [↩]
- Global Asthma Report 2022. International Journal of Tuberculosis and Lung Disease. (2022). [↩]
- M. Barne. Gaps in asthma diagnosis and treatment in low- and middle-income countries. Frontiers in Allergy. (2023). [↩]
- A. Inserro. American Journal of Managed Care. CDC study puts economic burden of asthma at more than $80 billion per year. The American Journal of Managed Care. (2023). [↩]
- C. Roduit, L. O’Mahony, et al. High levels of Butyrate and Propionate in early life are associated with protection against atopy. Allergy. (2019). [↩] [↩]
- P. Mahesh. Unraveling the role of ADAM33 in Asthma. National Institutes of Health. (2013). [↩]
- F. Guo, Y. Hao, L. Zhang, D. Croteeau-Chonka, D. Thibault, P. Kothari, L. Li, B. Levy, X. Zhou, B. Raby. Asthma Susceptibility Gene ORMDL3 Promotes Autophagy in Human Bronchial Epithelium. National Institutes of Health. (2021). [↩]
- N. Habib, M. Pasha, D. Tang. Current Understanding of Asthma Pathogenesis and Biomarkers. Cells, 11(17), 2764. (2022). [↩]
- P. Patel, S. Sharma. Bronchodilators. National Institute of Health. (2025). [↩]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. (2024). [↩] [↩]
- E.D. Bateman, H.K. Reddel, P.M. O’Byrne, et al. As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. New England Journal of Medicine. (2018). [↩]
- R. AbuJabal, R. Ramakrishnan, et al. The role of IL-5 in asthma and airway remodelling. Clin Exp Allergy (2019). [↩]
- W.W. Busse, J.F. Maspero, et al. Liberty Asthma QUEST: Phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy and safety in patients with uncontrolled, moderate-to-severe asthma. Lancet (2019). [↩]
- J. Radhakrishnan, B. Kennedy, E. Noftall, et al. Recent Advances in Phytochemical-Based Topical Applications for the Management of Eczema: A Review.Int J Mol Sci (2024). [↩]
- Mayo Clinic. Anti-interleukin-5 therapy for severe asthma: A new therapeutic option. Mayo Clinic. (2020). [↩]
- I.D. Pavord, S. Korn, P. Howarth, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. (2012). [↩]
- E.R. Bleecker, J.M. FitzGerald, P. Chanez, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β₂-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. (2016). [↩]
- M. Castro, J. Zangrilli, M.E. Wechsler, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. (2015). [↩]
- X. Dai, Y. Xu, H. Zhang. Advances in asthma research and treatment. PubMed Central. (2021). [↩]
- W. Busse, J. Corren, B.Q. Lanier, et al. Omalizumab, an anti-IgE monoclonal antibody, for the treatment of asthma. J Allergy Clin Immunol. (2001). [↩]
- A. Menzies-Gow, J. Corren, A. Bourdin, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. New England Journal of Medicine. (2021). [↩]
- A. Menzies-Gow, G. Colice, J.M. Griffiths G. Almqvist, et al. NAVIGATOR: a phase 3 multicentre, randomized, placebo-controlled trial of tezepelumab in adults and adolescents with severe uncontrolled asthma. Respir Res. (2020. [↩]
- X. Xiao, C. Schaefer, A. Szende, E. Genofre, et al. A cost comparison of benralizumab, mepolizumab, and dupliumab in patients with severe asthma. J Manag Care Spec Pharm. (2023. [↩]
- M. Wang, M. Schedel, E. Gelfland. Gene Editing in Allergic Diseases: Identification of Novel Pathways and Impact of Deleting Allergen Genes. J Allergy Clin Immunol (2024). [↩] [↩] [↩]
- S.F. Thomsen. Genetics of asthma: an introduction for the clinician. Eur Clin Respir J. (2015). [↩]
- C. Moses, P. Kaur. Applications of CRISPR systems in respiratory health. Respirology. (2019). [↩]
- D. Henry. Comparison of non-viral delivery vehicles for CRISPR/Cas9 genome editing. Stem Cell Reports. (2023). [↩]
- E. Rininella, P. Raoul, M. Cintoni, F. Franceschi, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. (2019). [↩]
- J. Chen, T. Li, C. Ye, J. Zhong, et al. The Lung Microbiome: A New Frontier for Lung and Brain Disease. Int J Mol Sci. (2022). [↩]
- X. Zhao, M. Hu, H. Zhou, Y. Yang, et al. The role of gut microbiome in the complex relationship between respiratory tract infection and asthma. Front Microbiol.(2022). [↩]
- P.G. Gibson, V.M. McDonald. Asthma-COPD overlap: now we are six. Thorax. (2017). [↩]
- J. Park, M. Kim, S. G. Kang, B. Cooper. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunology. (2015). [↩]
- A. Cait, M.R. Hughes, F. Antignano, J. Cait, et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids Mucosal Immunology. (2017). [↩] [↩]
- D.M. Patrick, H. Sbihi, D. Dai, A. Mamun, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. The Lancet Respiratory Medicine. (2020). [↩]
- C. Roduit, L. O’Mahony, et al. High levels of Butyrate and Propionate in early life are associated with protection against atopy. Allergy. (2019). [↩]
- Y. Chen, X. Li. Advances in asthma therapy and immunomodulation. ScienceDirect, Journal of Allergy and Immunology. (2023). [↩]






