Abstract
A solid tumor is an abnormal mass of uncontrolled cell growth, typically originating in the breast, lung, or prostate. Its physiological features make treatment by traditional methods difficult. In Solid tumors, the B7H4 receptor is overexpressed on the surface of the cancer cells, i.e., the number of receptors on cancer cells increases. This makes them an essential target for cancer diagnosis and targeted therapy. DuoBody is a bispecific antibody with two halves of antibodies designed to target two specific receptors and enhance therapeutic effects by bringing T cells to cancer cells, resulting in cancer cell apoptosis by the T cell. This study investigates B7H4 receptor overexpressed in solid tumors, and the CD3e receptor, which plays a role in activating T-cell response. I hypothesize that these DuoBody antibodies can be used to target the B7H4+ solid cancer cells by inducing the CD3+ T-cells toward the cancer cells. In the current research, I have performed computational modeling to design DuoBody antibodies targeting cancer (B7H4 receptor) and CD3+ T cells receptor). Initially, I got the 3D structures of the receptors by using the AlphaFold 3 web server. The 3D structure of antibodies was downloaded from the protein data bank. The downloaded antibodies were docked on the predicted receptor structures utilizing the HDOCK2.0 software to understand the antibodies’ binding interaction and affinity. The docking results were validated using the graph neural network (GNN). Graph Neural Network analysis was performed using the GRASP web server. Figure 2, shown in blue, displays the results from the GRASP server, with the predicted binding site highlighted in a box. The docking results confirmed that the antibody binds to this predicted site, thereby validating the docking outcomes. The antibodies were selected based on visual inspection, binding energy, and hydrogen bond interactions of the output obtained from the molecular docking simulations. The Binding energies calculated by the PRODIGY software showed that antibodies 4a6y and 2uyl strongly bind to the CD3e receptor and that 1il1 and 1f8t strongly bond to the B7H4 receptor. This research can be used in pharmaceutical drug development to engineer Duobodys targeting cancer cells.
Introduction
Solid tumors are abnormal masses of tissue that form in organs or tissues, typically composed of cancerous or non-cancerous cells, as opposed to blood-based cancers like leukemia.1 Cancerous solid tumors are the uncontrolled growth of cell tissues and can be either benign or malignant.2 Specifically, solid tumors are solid and don’t have any cysts or liquid areas.2 They are named for the types of cells they are made of (Ex, Bone, kidney, liver, and lung cancer).3 Solid tumors create a lump as they expand and are identified by screenings (mammograms, colonoscopies), biopsies, or blood tests(searching for tumor markers).3 Solid tumors can metastasize, or, spread to other parts of the body.4 Solid tumors are treated by surgery, radiation therapy, and chemotherapy, depending on the stage and type of the tumor. Detecting a solid tumor early significantly improves the survival rate of the patient (Therasse et al., 2000). B7H4 is a receptor highly expressed in various cell tumor types and is associated with tumor aggressiveness and lower survival rates.5 B7H4 is identified as a therapeutic target for the treatment of cancers.5 B7H4 negatively affects the immune response of T cells and inhibits their cell cycle, playing an essential role in tumor growth.5 Solid tumors are abnormal tissue growths that can invade nearby organs and spread throughout the body.6 Current treatments, such as surgery, chemotherapy, radiation, and targeted therapies, have improved survival but still face major limitations. These include a lack of specificity, leading to damage to healthy tissues and severe side effects, as well as the development of drug resistance over time.2 Additionally, many therapies struggle to reach or penetrate the tumor microenvironment effectively, and tumors can often evade immune detection, reducing the success of immunotherapy.6 To overcome these challenges, novel DuoBody antibodies are being developed. These bispecific antibodies bind both tumor-specific antigens and immune cells (such as CD3 on T-cells), helping to direct the immune system to attack cancer cells precisely. This dual-targeting approach enhances immune response, reduces off-target effects, and offers a promising new strategy for treating solid tumors resistant to conventional therapies.
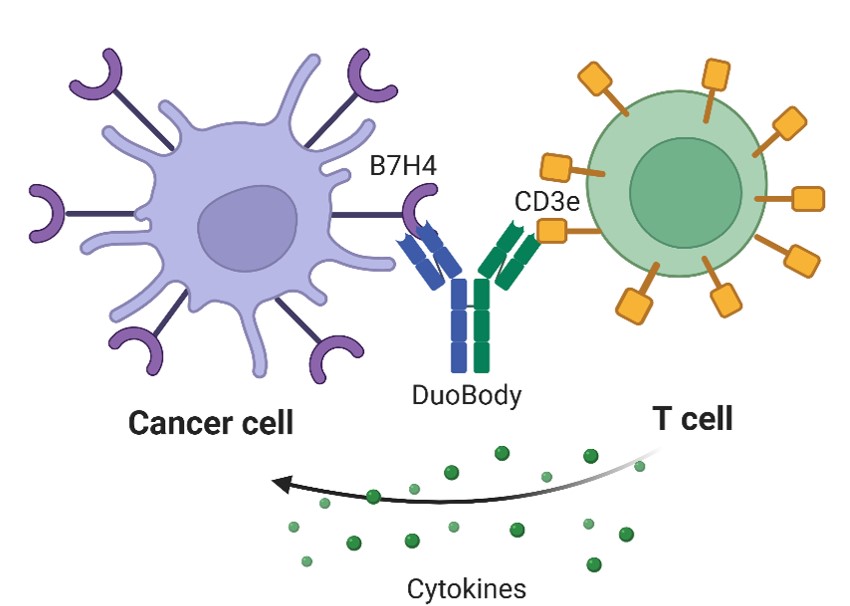
Antibodies are proteins in the bloodstream that are designed to discover antigens and trigger chemical reactions in the body to remove them.7 Antibodies are Y-shaped molecules with two antigen-binding sites at the two tips of the Y-shape.7 DuoBody is a platform for the development of bi-specific antibodies.8 DuoBodies consist of two halves of different antibodies designed to bind to two specific targets, triggering more of a particular chemical pathway.9 The dual-targeting properties of DuoBody enhance therapeutic potential, allowing the development of specific treatments for various diseases.9The schematic of the bispecific antibodies is shown in Figure 1.
Molecular docking is a computer program that predicts how molecules will interact with each other.10 In this scenario, molecular docking predicts how a ligand binds to the receptor.10Molecular docking works by running a search algorithm that generates possible binding models by finding each orientation of the ligand that fits into the receptor’s binding area.10 Then, the potential binding complexes are ranked based on the strength of interactions like hydrogen bonds and van der Waals forces.11 Molecular docking is used in pharmacology, specifically in structure-based drug design and drug effects calculation.10
Koopman et al. have designed bispecific antibodies targeting CD3e receptors present on the surface of T cells and B7H4 located on the solid tumor cancer cell.12 This opens up the opportunity to develop T cell-mediated solid tumor treatment. We hypothesize that these DuoBody antibodies can be used to target the B7H4+ solid cancer cells by inducing the CD3+ T-cells toward the cancer cells. In this research work, we have performed computational simulations to identify variable regions of antibodies specific to the two receptors. Our results suggest that 1IL1 against B7H4 and 4A6Y against CD3e receptor were selected. This research can be used in pharmaceutical drug development to engineer Duobody targeting cancer cells.
Method
This research paper used the B7H4 cancer receptor and the CD3e T cell receptor. The structure of these receptors was obtained by using AlphaFold3.13 The AlphaFold 3-predicted structures for B7H4 and CD3e showed high confidence in the proposed binding regions, with pLDDT scores above 85, and most ectodomain regions scoring ≥90, indicating reliable structural predictions for docking. Lower scores were noted in transmembrane areas, but these regions were not involved in binding. The CD3e structure aligned well with known antibody-bound structures (e.g., PDB: 4A6Y), supporting its accuracy.13 While no experimental structure exists for full-length B7H4, the predicted binding site matched conserved domains of the B7 family. Limitations remain due to the absence of experimental validation, and future structural studies are recommended. The structures of the antibodies used in this research paper were downloaded from the RCSB Protein Data Bank.(Rose et al., 2016) Next, the binding site on the receptors was predicted using the GrASP.11 GrASP is a graph neural network-based method to determine binding sites on protein surfaces [Figure 2]. Then, ESP (Electrostatic Surface Potential) was calculated using the ChimeraX v1.6 [Figure 2].14 Next, to understand receptor and antibody interactions, each receptor-antibody combination was modeled using the HDOCK2.0 [Figures 3 and 4].11 The HDOCK2.0 software uses a molecular docking and template-based modeling algorithm to simulate receptor-antibody binding complexes.11 HDOCK software, by default, performs protein-protein docking using a hybrid approach that combines template-based modeling and ab initio free docking. It accepts input in PDB format, automatically detects receptor and ligand chains, and removes water molecules or any HETATM records. The docking is rigid-body by default with no flexible residues considered, and it does not require predefined binding site information, enabling blind docking. The scoring function used is a knowledge-based potential (ITScore), and the top 100 docking models are generated and ranked. Clustering is applied to reduce redundancy in predicted poses. If homologous templates are available, HDOCK automatically uses them to enhance accuracy; otherwise, it defaults to free docking. It supports protein-DNA/RNA interactions but does not handle small-molecule docking. Next, the binding energy in these complexes was computed using the PRODIGY software.15 Figures were generated using BioRender (2024 version) and ChimeraX (v1.6) software.14 This study focused on antibodies with potential binding to B7H4 and CD3e; however, no negative control antibodies—known not to bind these receptors—were included. For future validation of the pipeline’s predictive accuracy, it is essential to incorporate such controls. These would help confirm the specificity and reliability of the docking and scoring process. Including non-specific antibodies (e.g., anti-GFP or anti-insulin antibodies) in the same computational workflow should yield higher binding energies (e.g., > -9.0 kcal/mol) and poor alignment with predicted binding sites, thereby reinforcing the model’s ability to discriminate between true and false binders. This step will enhance the robustness of the in silico screening pipeline before any experimental validation.
Results
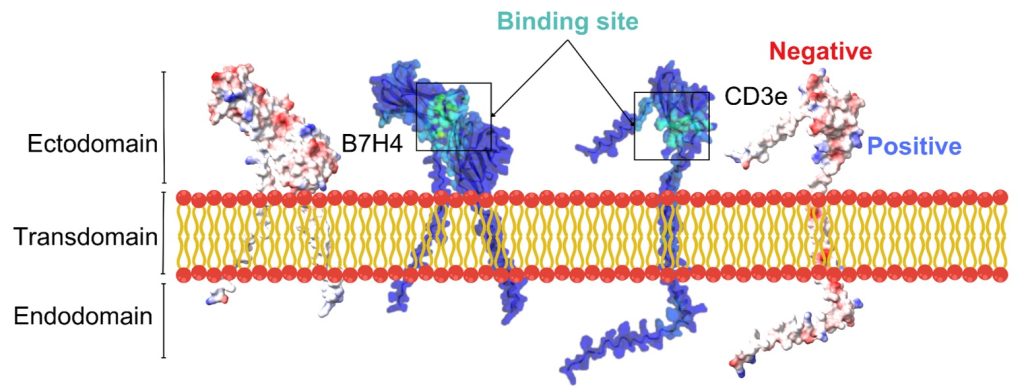
Receptor Structure Prediction: To start the research, we first obtained the 3D structure of the receptor using AlphaFold 3. It is an AI model developed by Google DeepMind and Isomorphic Labs that accurately predicts the structure of proteins, DNA, RNA, and ligands. These receptors are present on the surface of the cell and have three regions: i.e., an ectodomain (outside the cell), an endodomain (inside the cell), and a transdomain (across the plasma membrane), Figure 2. To understand the surface binding properties of these receptors, we used a graph neural network analysis method called GrASP. It is a graph neural network tool used to accurately find the optimal binding sight for the ligand to the receptor. Electrostatic surface potential is a three-dimensional map showing a molecule’s charged regions. ESP is used in drug design to optimize interactions between proteins and ligands. The ESP of the B7H4 and CD3e proteins plays a crucial role in identifying biologically active regions, particularly the binding sites targeted by antibodies. In the image, the boxed “Binding site” regions are characterized by distinct charged surface patches, which are often indicative of ligand or antibody interaction zones. These regions likely exhibit complementary charges that promote strong and specific antibody binding. This electrostatic complementarity is essential in the design of bispecific antibodies targeting B7H4 and CD3e for solid tumor therapy, as it enhances binding affinity and specificity. Furthermore, the charge distribution observed in these regions provides functional validation for the docking results, confirming that the antibodies bind in areas of charge compatibility. This supports the credibility of the computational model and reinforces its potential utility in developing effective targeted cancer treatments.
Molecular Docking Simulation: In the next step, the receptor antibody molecular docking simulations were performed using the HDOCK 2.0 web server to get the receptor-antibody complex structure. The docked structure is displayed in Figures 3 and 4. The antibodies were selected by visual inspection using the ChimeraX software, where the complex was chosen based on whether or not it bound to the receptor in the binding site and then if the orientation of the antibody had a coil facing away from the binding site. These antibodies were further selected for the following selection process. In the next step, we also performed the receptor-antibodies binding affinity calculations. Binding energy measures the smallest amount of energy required to move a particle. The binding energy is measured in kcal/mol, and the most negative binding energy number is the most robust binder. PRODIGY by Bovin Lab is a website designed to predict the binding affinity in biological complexes based on the structural properties of the protein-protein complexes. The binding energy of the antibodies is shown in Table 1, and among the five B7H4 selected antibodies, 1il1 has the highest binding affinity (-13.7 kcal/mol). On the other hand, 4A6Y had the highest binding affinity (-16.1 kcal/mol) for the CD3e. The molecular docking simulations of antibody interactions with B7H4 and CD3e receptors revealed key structural insights supporting bispecific antibody design. In the B7H4 panel (Figure 3), antibodies 1b4j, 1il1, 2hrp, 1nlb, and 1iqw demonstrated binding at or near the predicted receptor binding site, suggesting their potential to effectively recognize and engage B7H4-expressing tumor cells. Similarly, docking results for CD3e (Figure 4) showed that antibodies 1b4j, 1nlb, and 1a5f consistently interacted with the functional binding region on the CD3e receptor, indicating their capability to activate T cells through CD3 engagement. Notably, antibody 1b4j exhibited favorable binding to both B7H4 and CD3e, highlighting it as a promising candidate for bispecific antibody development. These findings validate the structural compatibility and binding specificity of selected antibodies, providing a foundational step toward engineering dual-target immunotherapeutics for solid tumor treatment.


Table 1 shows the predicted binding energies between selected antibodies and the B7H4 and CD3e receptors. Binding energy, measured in kcal/mol, reflects the strength of interaction between an antibody and its receptor—the more negative the value, the stronger the binding. Among the antibodies tested, 1f8t exhibited the strongest binding to the B7H4 receptor with a binding energy of -17.1 kcal/mol, while 4a6y showed the strongest affinity for the CD3e receptor at -16.1 kcal/mol. Antibodies such as 1il1 and 1fl5 also demonstrated strong binding to both receptors, with values around -14.0 kcal/mol, making them strong candidates for bispecific antibody design. In contrast, antibodies like 1a5f and 2hrp displayed relatively weaker binding to CD3e, suggesting they may be less effective for T cell engagement. Overall, the data highlights 1f8t, 4a6y, and 1il1 as promising leads for engineering DuoBody antibodies targeting solid tumors.
| Binding energy (kcal/mol) | ||
| Antibodies name | B7H4 | CD3e |
| 1a5f | -13.9 | -10.7 |
| 1b4j | -12.6 | -12.9 |
| 1fl5 | -13.5 | -14.0 |
| 1il1 | -13.7 | -14.0 |
| 1iqw | -12.8 | -14.0 |
| 1nlb | -12.9 | -11.6 |
| 2hrp | -12.3 | -12.0 |
| 4a6y | -11.1 | -16.1 |
| 1f8t | -17.1 | -14.4 |
| 2uyl | -13.6 | -15.9 |
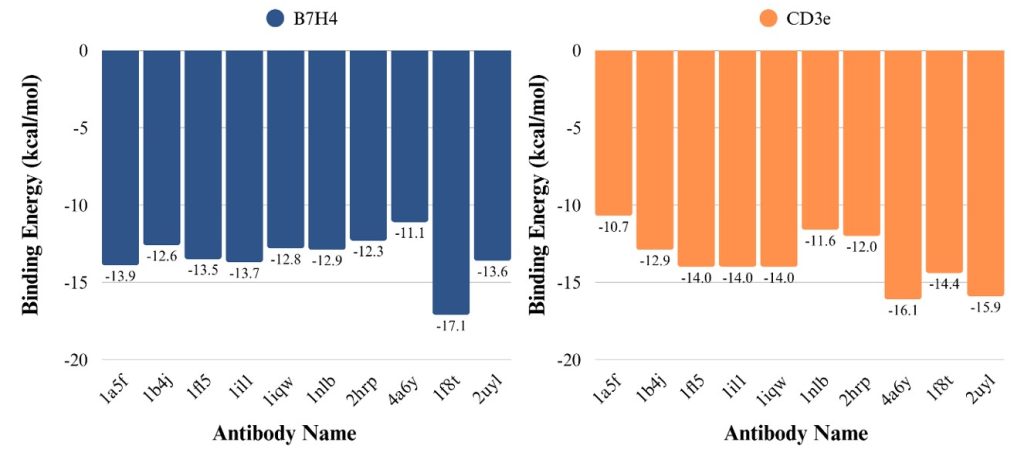
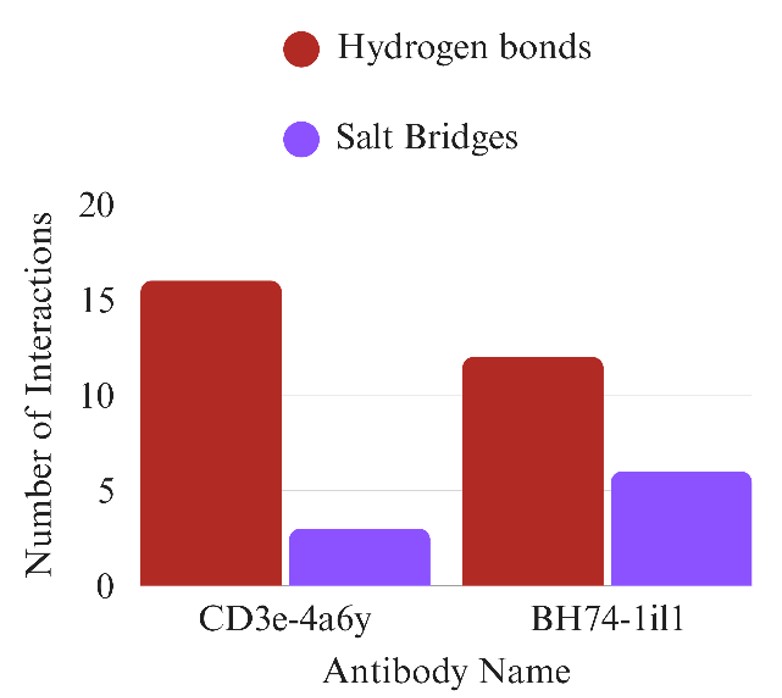
Figure 6 demonstrates the binding affinity patterns of several antibodies to the B7H4 and CD3e receptors, key targets in cancer immunotherapy. Antibodies such as 1b4j, 1il1, 1nlb, and 1a5f show strong binding (more negative scores), suggesting they may be promising candidates for bispecific antibody design. The variation in docking scores highlights the importance of structure-specific interactions. Comparative analysis reveals which antibodies may serve as effective dual binders, supporting the rationale for using computational docking in antibody screening for solid tumor therapies.
This line graph shows the docking scores of multiple antibodies (x-axis) when docked against the B7H4 (orange line) and CD3e (blue line) receptors. Lower docking scores indicate stronger binding affinities. Each antibody is represented with a corresponding docking score for both targets.
Discussion
While traditional therapies like surgery, chemotherapy, and radiation remain standard treatments for solid tumors, they often lack specificity and can cause significant off-target effects.16 This study provides a computational framework for designing bispecific DuoBody antibodies that simultaneously target B7H4 on tumor cells and CD3e on T cells, bridging the immune system directly to the cancer. By identifying antibody candidates with strong predicted binding affinity and structural compatibility, we demonstrate how AI-based modeling and docking can inform rational design of next-generation immunotherapies. These findings offer a targeted alternative to conventional approaches, with the potential to overcome immune evasion and enhance tumor-specific cytotoxicity — a critical advancement for hard-to-treat solid tumors.
One conventional solid tumor treatment strategy is resection, removing the tumor and some surrounding healthy tissues. Sometimes, if the entire region cannot be removed, a significant portion of the cancer tissue is removed to help reduce the subsequent treatment. Other types of cancer therapy include chemotherapy, radiation therapy, and CD3+ T-cell therapy. Chemotherapy and radiation therapy work by damaging cancer cells so that they cannot go through the cell cycle and divide. CD3+ T-cell therapy is more effective for leukemia and melanoma and assists the patient’s T-cells in fighting cancer. This study focused on receptor-specific binding but did not assess potential off-target interactions with receptors on healthy cells. Future work should include whole-proteome docking or cross-reactivity analysis to evaluate non-specific binding, especially since B7H4 may have low-level expression in normal tissues. Minimizing off-target effects is essential to ensure therapeutic safety in clinical applications.
Application and Limitations: This research can be used for targeted cancer therapy. In targeted therapy, only the specific cancer cell is destroyed, leaving the healthy or normal cells unharmed.17 Because the B7H4 receptor is more common in solid tumors, this research applies explicitly to treatment for solid tumors.5 In addition to treating cancer cells, the DuoBody antibody epitope can also be used to diagnose cancer when the fluorescent dye is attached to the DuoBody and binds to the cancer cell.18 Since the antibodies and receptors are inside the body, they are surrounded by water. In future studies, we will conduct molecular dynamics simulations to ensure the antibody remains bound to the receptor surface. Since the research is computational, the next step would be to perform experimental validation. Lab experiments like ELISA (Enzyme-Linked Immunosorbent Assay) will be used to find the binding strength of the antibody.19 In addition, due to the limited number of antibodies screened in the study, other antibodies not considered in this study could be more effective than the ones shown in our results.
Conclusion
This study identified antibody candidates 1il1 and 4a6y as strong binders to B7H4 and CD3e receptors, respectively, suggesting their promise for DuoBody design in targeted cancer therapy. While the results demonstrate favorable binding through computational simulations, they remain predictive. Experimental validation through in vitro assays, such as ELISA or flow cytometry, is essential to confirm specificity and binding affinity. Therefore, while this research offers a valuable framework for bispecific antibody development, its preliminary conclusions must be interpreted within the scope of in silico limitations.
References
- Travis, L. B. (2002). Therapy-associated Solid Tumors. Acta Oncologica, 41(4), 323-333. [↩]
- Travis, L. B. (2002). Therapy-associated Solid Tumors. Acta Oncologica, 41(4), 323-333 [↩] [↩] [↩]
- Ramaswamy, S., Ross, K. N., Lander, E. S., & Golub, T. R. (2003). A molecular signature of metastasis in primary solid tumors. Nature Genetics, 33(1), 49-54 [↩] [↩]
- DeNardo, D. G., Johansson, M., & Coussens, L. M. (2008). Immune cells as mediators of solid tumor metastasis. Cancer and Metastasis Reviews, 27(1), 11-18 [↩]
- Dawidowicz, M., Kot, A., Mielcarska, S., Psykała, K., Kula, A., Waniczek, D., & Świętochowska, E. (2024). B7H4 Role in Solid Cancers: A Review of the Literature. Cancers (Basel), 16(14). [↩] [↩] [↩] [↩]
- Therasse, P., Arbuck, S. G., Eisenhauer, E. A., Wanders, J., Kaplan, R. S., Rubinstein, L., . . . Gwyther, S. G. (2000). New Guidelines to Evaluate the Response to Treatment in Solid Tumors. JNCI: Journal of the National Cancer Institute, 92(3), 205-216 [↩] [↩]
- Forthal Donald, N. (2014). Functions of Antibodies. Microbiology Spectrum, 2(4), 10.1128/microbiolspec.aid-0019-2014 [↩] [↩]
- Engelberts, P. J., Hiemstra, I. H., de Jong, B., Schuurhuis, D. H., Meesters, J., Beltran Hernandez, I., . . . Breij, E. C. W. (2020). DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. eBioMedicine, 52 [↩]
- Kontermann, R. E., & Brinkmann, U. (2015). Bispecific antibodies. Drug Discovery Today, 20(7), 838-847 [↩] [↩]
- Fan, J., Fu, A., & Zhang, L. (2019). Progress in molecular docking. Quantitative Biology, 7(2), 83-89 [↩] [↩] [↩] [↩]
- Yan, Y., Tao, H., He, J., & Huang, S.-Y. (2020). The HDOCK server for integrated protein–protein docking. Nature Protocols, 15(5), 1829-1852 [↩] [↩] [↩] [↩]
- Koopman, L., Smits-de Vries, L., Egerod, F. L., Wubben, S., Houtkamp, M., De Poot, S., . . . Verzijl, D. (2021). 863 In vitro and in vivo studies establish DuoBody®-CD3xB7H4 as a novel drug candidate for the treatment of solid cancers. Journal for ImmunoTherapy of Cancer, 9(Suppl 2), A904-A904 [↩]
- Abramson, J., Adler, J., Dunger, J., Evans, R., Green, T., Pritzel, A., . . . Jumper, J. M. (2024). Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature, 630(8016), 493-500 [↩] [↩]
- Pettersen, E. F., Goddard, T. D., Huang, C. C., Meng, E. C., Couch, G. S., Croll, T. I., Ferrin, T. E. (2021). UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein science, 30(1), 70-82 [↩] [↩]
- Vangone, A., & Bonvin, A. (2017). PRODIGY: A Contact-based Predictor of Binding Affinity in Protein-protein Complexes. Bio Protoc, 7(3), e2124 [↩]
- Luo, D., Carter, K. A., Miranda, D., & Lovell, J. F. (2017). Chemophototherapy: An Emerging Treatment Option for Solid Tumors. Advanced Science, 4(1), 1600106 [↩]
- Tsimberidou, A.-M. (2015). Targeted therapy in cancer. Cancer Chemotherapy and Pharmacology, 76(6), 1113-1132 [↩]
- Conroy, P. J., Hearty, S., Leonard, P., & O’Kennedy, R. J. (2009). Antibody production, design and use for biosensor-based applications. Seminars in Cell & Developmental Biology, 20(1), 10-26 [↩]
- Bobrovnik, S. A. (2003). Determination of antibody affinity by ELISA. Theory. Journal of Biochemical and Biophysical Methods, 57(3), 213-236 [↩]







