Abstract
Background: Triclosan is a common antibacterial ingredient in pharmaceutical and personal care products that has emerged as a persistent pollutant harming marine ecosystems. Once triclosan enters our wastewater systems, it is degraded into compounds that are more toxic than the parent compound in some cases. Current removal methods rely on costly advanced oxidation processes. Recently, microalgae-based remediation of triclosan has attracted interest due to its cost-effective, efficient and flexible approach.
Methods: Removal agents used in this work include the immobilized form of freshwater microalgae strains (Chlorella pyrenoidosa and Scenedesmus quadricauda) and clay minerals (montmorillonite and clinoptilolite). Changes in triclosan concentrations over 0-120 hours were monitored using gas chromatography-mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC). An environmentally relevant concentration of triclosan (800 ng/mL) was used in the studies.
Results: The percent reduction in triclosan was recorded when immobilized microalgae beads (mono-culture, co-culture) were incubated with triclosan. The best performing experimental groups reduced triclosan by 88% in 6 hours (C. pyrenoidosa alone) and by 48-69% in 4 hours (co-culture algae beads protected from the light). Supplementation of the co-culture algae beads with clay minerals provided no enhancement at each time point in the dark.
Conclusions: Despite their preliminary nature, our findings suggest that immobilized algae beads can remove triclosan within a short timeframe and that clay minerals provide little enhancement. This work contributes to the ongoing search for diverse materials for triclosan remediation from wastewater.
Keywords: triclosan, bioremediation, wastewater, immobilized microalgae, Chlorella pyrenoidosa, Scenedesmus quadricauda, clay minerals, montmorillonite, clinoptilolite.
Introduction
Background and Context
Triclosan is an antibacterial agent common in pharmaceutical and personal care products like soap and toothpaste1. Extended exposure to triclosan for humans can lead to trace amounts in breast milk and urine1. Existing wastewater treatment facilities use activated bacterial sludge treatment, advanced oxidation processes, and adsorption onto activated carbon to remove triclosan; these processes remove 58-98% of the triclosan but are costly and difficult to maintain2,3. Approximately 58% of U.S. river water samples contain triclosan2. This antibiotic can also be found in both the water and the sediment of marine environments where it acts as an endocrine disruptor, a carcinogen of animal models and toxic to marine organisms2,4. The persistence of this antibiotic in the environment also increases bacterial resistance to it. The triclosan-loaded sludge poses risks to the environment and requires additional disposal. Triclosan is highly photosensitive and degrades into harmful chlorinated by-products during exposure to light or during conventional wastewater processing5,6.
Chemical Properties of Triclosan
Triclosan is a chlorinated aromatic compound with a half-life of 2.55 hours in sunlight when suspended in water1. It is hydrophobic at acidic pH values and hydrophilic at basic pH values due to the pKa of 8.14 for the hydrogen of the hydroxyl functional group. These properties complicate triclosan removal strategies because the dominant form (protonated vs. ionized) depends on the pH of the surrounding solution1,7. This means that the relative amount of triclosan vs. ionized triclosan is 50/50 at pH 8.14, which warrants consideration given that the pH of wastewater varies from 7.0-8.78. However, in a neutral solution at pH 7, the dominant form of triclosan is the nonpolar triclosan, making up 92.6% of the triclosan concentration8. Efforts to remediate triclosan from wastewater would ideally target both nonpolar and ionized forms of triclosan.
Public Policies on Triclosan
Because of the threat triclosan poses to marine ecosystems and humans, many countries have taken legal action concerning the use of triclosan in products. As early as 2011, many companies that sell personal-care products have discontinued their use of triclosan as well9. These companies include Procter & Gamble, Johnson & Johnson, and Colgate-Palmolive. In 2013, the U.S. Food and Drug Administration (FDA) revoked a previous statement made about the safety of triclosan’s presence in hand soap and called for additional research into health effects and possible amplification of bacterial resistance10. Three years later in 2016, the FDA ruled that 19 personal care product ingredients containing triclosan were unsafe, ineffective, and misbranded11. Companies were required either to change their recipe, no longer sell their products, or submit a New Drug Application in order to continue selling their products that contained any of these 19 ingredients. In 2016, the European Commission did not approve the use of triclosan as an active substance in antimicrobial products12.
Microalgae Remediation Mechanisms and Immobilization
Freshwater microalgae in planktonic form can remove pollutants from wastewater through three different mechanisms7,13. First, bioadsorption allows the cell wall of the microalgae to collect substances from aqueous solution and adhere pollutants to its surface. The efficacy of this process differs depending on the characteristics of the contaminant, such as its polarity and chemical structure. One significant aspect of bioadsorption is that it does not break down the pollutant. A second mechanism involves bioaccumulation, which takes place when the cells uptake the pollutant from the wastewater along with other substances that may be present in the media. Lastly, biodegradation may occur within the cell after the pollutants are accumulated during the previous steps; microalgal enzymes degrade the pollutants into smaller molecules.
Algae-based remediation has many advantages, because the algae can tolerate many conditions, are self-sustaining through photosynthesis, and can be immobilized in alginate beads to facilitate separation from the wastewater media14. Immobilization of the microalgae increases its surface area, leading to a faster rate of bioremediation for all pollutants13. The microalgae is immobilized by trapping the algae in a hydrophilic polymer that acts as a physical barrier between the microalgae and the wastewater containing the pollutant15. The polymer encasing the microalgae contains pores that allow the wastewater to diffuse through the membrane and interact with the microalgae within the bead. The pollutant either adsorbs to, bioaccumulates within or is enzymatically degraded by the microalgae into biodegradation products. The algae bead is responsible for removing the contaminant, and the resulting “clean water” can be separated from the beads in a filtration step.
Freshwater Strains that Remove Triclosan
Chlorella pyrenoidosa is a freshwater green algae commonly used to remove pollutants16,17. C. pyrenoidosa has been an effective remediator of heavy metals and pharmaceuticals from aqueous solutions14,18. When targeting triclosan, C. pyrenoidosa relies primarily on uptaking triclosan into its cells (bioaccumulation) rather than metabolizing it via biodegradation19. Removal percentages range from 62% after 1 day to 69% after 7 days (initial concentration of 800 ng/mL)19. Most of the triclosan (59%) accumulated within the cells after 1 day but the high triclosan concentration inhibited microalgal growth19. Other reports showed that the strain tolerated a range of triclosan concentrations (100-800 ng/mL), of which 50% was removed from solution through cellular update during the first 1 hour of incubation20. A removal percentage of 77% was achieved after 96 hours of incubation with C. pyrenoidosa (800 ng/mL initial concentration)20.
Scenedesmus quadricauda is a second freshwater strain used to remove triclosan; it has a high growth rate, low nutrient requirements, and tolerance to heavy metals21. S. quadricauda was part of a consortium of microalgae and bacteria that achieved 79% removal of triclosan within 5 days; however, the initial triclosan concentration (8000 ng/mL) was toxic to the consortium after those 5 days22. Scenedesmus obliquus, a similar strain to S. quadricauda, achieved a 99.7% removal rate of triclosan within one day19. In contrast to C. pyrenoidosa, S. obliquus primarily biodegrades triclosan. The uptake of triclosan by S. obliquus cells was minimal, with only 2.1% absorbed in the first day and 1% after seven days, indicating that degradation rather than accumulation was the primary removal method19. Toxicity of triclosan (at 8000 ng/mL) to either microalgae after 5-8 days of incubation may limit the application of microalgae-based bioremediation22. Since Scenedesmus strains tolerate municipal wastewater contaminants better than Chlorella strains, co-culturing these strains for use in triclosan remediation may be a compelling strategy23.
Clay Materials that Remove Triclosan
Clay minerals have surface areas that absorb pollutants in solution, and several have been evaluated for triclosan removal24,25. Clinoptilolite is a naturally abundant high-silica zeolite that removes 90-99% of triclosan from water without any loss of efficiency over several cycles when the initial triclosan concentration is 8,000 ng/mL26,27. Montmorillonite is a clay mineral that is a common sorbent for industrial dyes because of its high surface area and absorption capacity28,29. Montmorillonite absorbed approximately 85% of triclosan (initially 100 ng/mL) at pH 4.7 and and 50% at pH 8.4 after 24 hours, demonstrating how pH can affect removal efficiency24. Moreover, at low pH values, less montmorillonite was needed to remove comparable amounts of triclosan, presumably due to the increased hydrophobicity of triclosan at low pH. For these reasons, clay minerals may be a useful supplement to bioremediation methods, depending on the pH optimized for microalgae viability.
Problem Statement and Rationale
While planktonic microalgae (C. pyrenoidosa and S. quadricauda) have been shown to be effective individual bioremediators of triclosan, the co-culture of these two strains immobilized in alginate beads might yield has not been evaluated. Our research posed these questions: What is the triclosan removal percentage for co-culture of two effective strains that have been immobilized into alginate beads? How does this compare with empty alginate beads? What is the effect of supplementing the algae bead cultures with clay minerals known to remove triclosan?
Significance and Purpose
No study has been published on the triclosan remediation capacity of immobilized mono-culture and co-culture of microalgae incubated with triclosan. The significance of this study is the prospect of identifying if algae beads pose a viable bioremediation strategy. Configuring different remediation agents in combinations could exploit the available mechanisms (adsorption to clay, bioadsorption to algae cells, bioaccumulation inside algae cells and biodegradation by algae enzymes). These combinations might lead to triclosan reductions on short timescales (hours, days). The practical application of this work is to develop renewable, flexible and cheaper methods for removing antibiotics that might be superior to conventional advanced oxidation processes.
Objectives
The main objectives of the study are:
- To track the concentrations of triclosan in the presence of 2 types of microalgae beads over 120 hours.
- To explore any synergistic effects of combining clay minerals and co-cultured microalgae beads at neutral pH on triclosan removal.
Scope and Limitations
This is a preliminary exploration of new combinations of triclosan removal agents using analytical methods available to students. The degradation of triclosan in aqueous media has been characterized by other groups using gas chromatography-mass spectrometry (GC-MS) and high performance liquid chromatography-mass spectrometry (HPLC-MS) and is not the focus of this work. Encouraging HPLC results are presented about the percent triclosan reductions within hours of incubating with new combinations. These findings warrant further confirmation with mass spectrometry, which is not part of the current study.
Methodology Overview
Experimental studies (12-24 flasks, experimental duplicates, 3-6 times points each, 0-120 hours) were conducted to study the ability of algae beads and clay minerals individually and in combination to remove triclosan in the presence/absence of light (Figure 1). The focus was on rapid removal (0-24 hours) and on conditions that approximated actual wastewater treatment facilities (pH 7-8). The bead concentration (4 beads/mL) and an environmentally relevant concentration of triclosan (800 ng/mL) were selected based on literature studies19,20.
The two analytical methods used during these studies were gas chromatography-mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC). The benefit of GC-MS was the identification of the components in each sample by molecular weight, namely of the target molecule triclosan. However, the GC-MS instrument was no longer available for use after Experiment A. Thereafter, HPLC was used as the primary analytical method. Relying on HPLC to track relative changes in triclosan concentration reduced the environmental impact of dichloromethane used in GC-MS analysis. Direct injection of aqueous samples onto the HPLC column also significantly reduced the sample preparation time compared to GC-MS.
Materials and Methods
Media, Chemicals and Instruments
- Media and Chemicals: municipal wastewater was obtained from the La Canada Water Reclamation Plant of the Los Angeles County Sanitation District. Chemicals were purchased from Sigma: HEPES, Sigma H4034-25G; triclosan, Sigma 647950-1GM-M; calcium chloride, C5670-100G. Clay minerals were obtained from the Clay Minerals Society: SWy-3, Na-rich Montmorillonite; KGa-2, Kaolinite. Zeolite (clinoptilolite, NAu-1) was sourced from Sweetwater County, Green River, Wyoming. Other chemicals such as methanol and dichloromethane were HPLC-grade.
- Immobilized Microalgae: Alginate beads containing Chlorella pyrenoidosa, Scenedesmus quadricauda and co-cultures of these two strains were purchased from Algae Research Supply (ARS), Vista, CA. Empty beads (containing water only, no algae) were provided by ARS for comparison.
- Analytical Instruments: Triclosan concentrations were determined using a research-grade GC-MS system (Agilent 7809A GC, equipped with an Agilent DB-5MS UI column (30m, 0.250 mm diameter, 0.25 um film) or HPLC system (Agilent 1100 Series equipped with an Agilent Eclipse XDB-C18 column [3.5 um, 2.1 x 100 mm], diode array detector set to 280 nm).
- Laboratory Equipment: Experimental flasks (sterile 50 mL square tissue culture flasks) were incubated on shakers (100 rpm) housed in bench-top grow tents equipped with a 25W-equivalent LED light, light timer (12:12 on:off), fan, and thermometer. Standard laboratory tools such as an analytical balance, micropipettes, speed vacuum, centrifuge and alcohol burners were used throughout the studies.
Media and Material Preparation
Air was bubbled through the wastewater to off-gas the chlorine present as obtained directly from the Los Angeles County Sanitation District. For the microalgae studies, HEPES was added to the wastewater to reach a final concentration of 10 mM HEPES and the pH was adjusted to 7.2. For the clay/microalgae studies, a solution of 20 mM calcium chloride was prepared at pH 7.2. All types of media were filter-sterilized through 0.2 micron filters and handled aseptically throughout the studies. A stock solution of triclosan was prepared in methanol, protected from light at all times and stored at 4 C prior to use. Clay was conditioned for 20-24 hours at room temperature in calcium chloride (20 mM, pH 7.2) without shaking prior to the studies. This step saturates the clay surfaces with calcium to eliminate any variability of the original clay materials prior to the addition of triclosan.
Incubation Studies
For the microalgae studies, 50 mL square flasks contained triclosan (800 ng/mL), wastewater media (10 mM HEPES, pH 7.2 or 20 mM CaCl2, pH 7.2), 40 algae beads (4 beads/mL), and clay (2.5 mg/mL of montmorillonite or clinoptilolite) where noted. Microalgae beads were counted on sterile surfaces and transferred aseptically to each experimental flask. Flasks were exposed to 12 hours of light per 24-hour period. Half of the experimental flasks were covered in foil to evaluate the role of light on triclosan removal. Each experimental condition was prepared in duplicate. Samples removed for analysis were protected from light at all times.
For Experiment A, the total volume of each flask was 30 mL. Aliquots (1 mL) were aseptically removed from each experimental flask at individual time points (0, 1, 6, 24, 48, 96 hours), evaporated to dryness, resuspended in dichloromethane and centrifuged. Eight hundred microliters (800 uL) were transferred to amber vials and analyzed by GC-MS. For Experiment B, the total volume of each flask was 10 mL and the results were analyzed by HPLC. Aliquots (300 uL) were removed aseptically from each experimental flask at individual time points (0, 1, 6, 24, 48, 120 hours) and centrifuged for 5 minutes at high speed. Supernatants (250 uL) were transferred to sleeved amber vials and analyzed immediately using HPLC. This workup omitted lengthy evaporation and resuspension steps required by GC-MS, as well as the use of dichloromethane.
Analytical Methods
Standards and samples containing known amounts of triclosan (0-1000 ng/mL) were prepared in media types, evaporated to dryness and then resuspended in dichloromethane or methanol to create calibration curves for triclosan determination (Supplementary Material). For GC-MS analysis, the standards were used to create a library of compounds to identify the composition of the experimental samples, and calibration curves were linear over the range of 80-1000 ng/mL triclosan with a R2 value of 0.997 (Supplementary Material). For HPLC analysis, peak areas of triclosan produced by the known standards were selected manually, and the resulting calibration curve was linear over the range of 100-900 ng/mL of triclosan with a R2 value of 0.996 (Supplementary Material). Media-only samples were injected to confirm peaks absorbing at 280 nm, the wavelength selected for the diode array detector for triclosan. Experimental samples were normalized to the t = 0 values for each respective group; the variability of these points was 11% (Supplementary Material). For a signal intensity (i.e., peak area) that is less than the t = 0 value, it was referred to as a reduction in triclosan concentration. Time points were expressed as a percent of triclosan remaining relative to the t = 0 value for that experimental group.
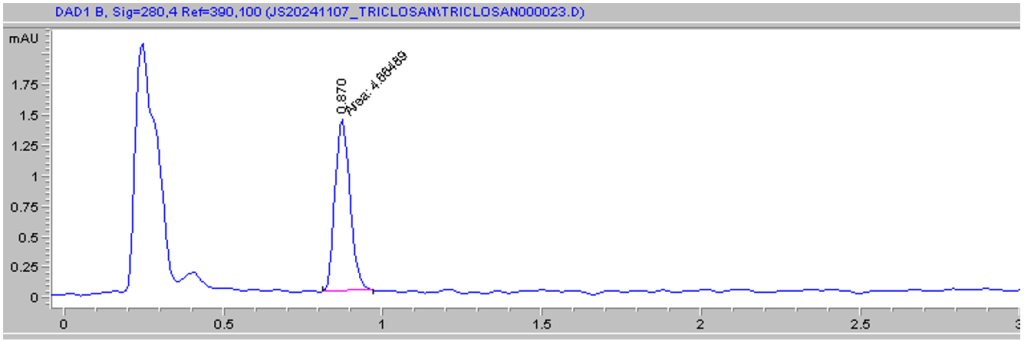
The GC-MS protocol used in the study was based on literature reports and was refined as follows: 70 C for 1 minute, 15 C/minute until 320 C, then 5 minutes at 320 C. Injection volume was 1.0 uL and the inlet temperature was 280 C1. The carrier gas was helium flowing at 1.109 mL/min at 10.055 psi. The HPLC protocol used in the study was based on literature reports and was refined as follows: mobile phase of 70/30/0.1% acetonitrile/water/acetic acid at 1.0 mL/min using an isocratic gradient6. Injection volume was 40 uL; column temperature was 25 C, and the method run time was 5.0 minutes. Triclosan consistently eluted at t = 0.87 minutes and has a lambda max of 280 nm in the solvent system (Figure 2).
Results
Experiment A
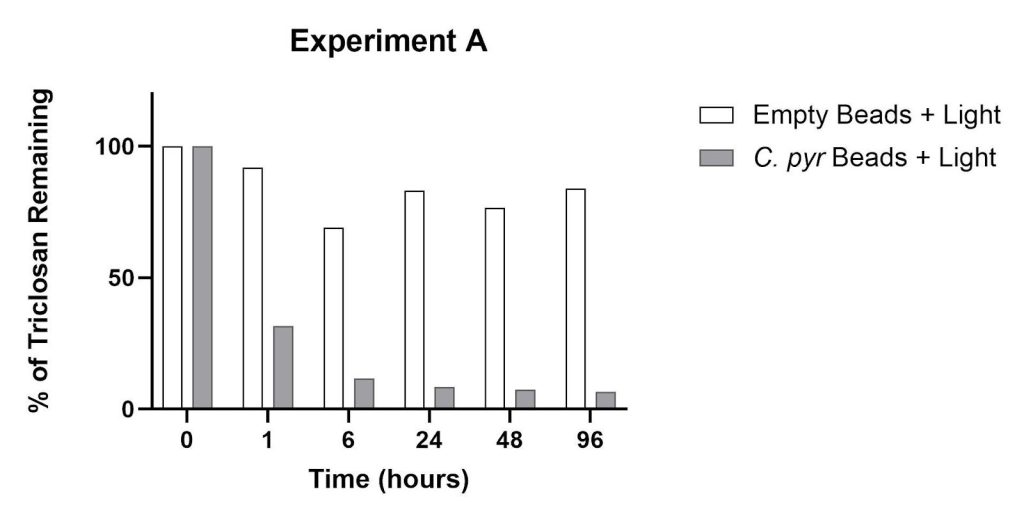
The presence of a single species (C. pyrenoidosa) inside alginate beads was evaluated for its effect on triclosan removal over a period of 96 hours, compared to alginate beads containing no microalgae (Figure 3). Within 1 hour of incubation, the amount of triclosan in the presence of algae beads was reduced by 68% compared to 8% in the presence of empty beads. After 6 hours, triclosan was reduced by 88% due to the C. pyrenoidosa beads. After 24 hours of incubation, 92% of the triclosan was removed by the algae beads compared to 24% by the empty beads. Compared to the 24-hour data, the percent reductions at 96 hours for both experimental groups remained unchanged.
Experiment B
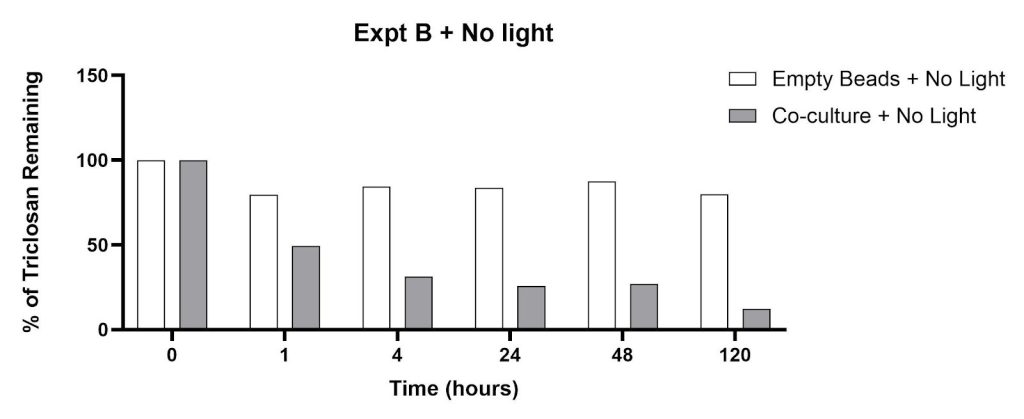
This experiment explored the triclosan removal capacity of beads containing a co-culture of C. pyrenoidosa and S. quadricauda in the presence and absence of clay minerals (montmorillonite, clinoptilolite). Similar to Experiment A, the empty beads produced minimal remediation after 120 hours: 16% concentration reduction with light, 20% concentration reduction in the dark (Figures 4 and 5). The co-culture/dark conditions led to significant percent reductions within 1-24 hours as shown in Figure 4: 51% (1 hour), 69% (4 hours), 74% (24 hours), 73% (48 hours) and 88% (120 hours). The co-culture/light experimental groups showed an increase in signal intensity for time points at 1, 4, and 24 hours (Figure 5). The percent triclosan reductions for the co-culture/light flasks were 23% (48 hours) and 68% (120 hours).
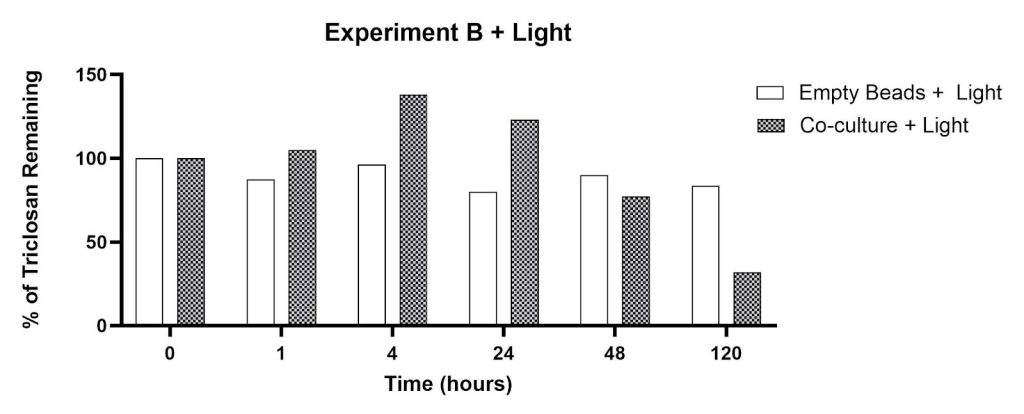
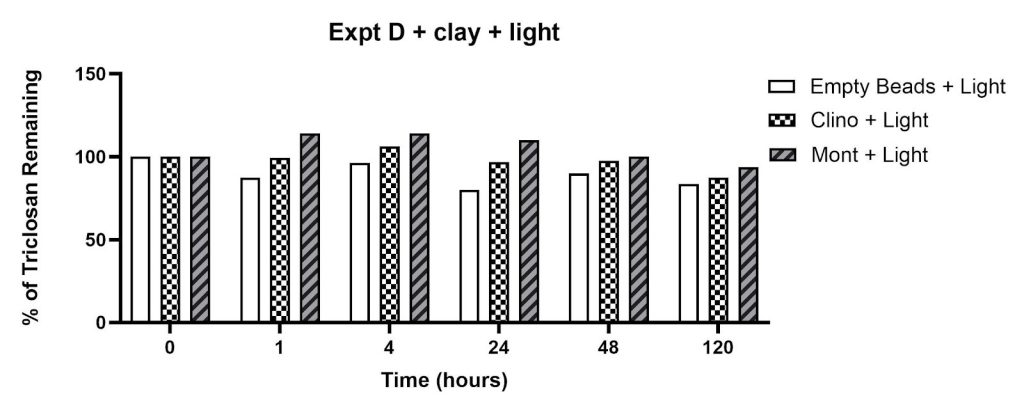
The effect of clay minerals on triclosan removal is shown in Figures 6 and 7. The percentage of triclosan remaining was observed in flasks containing (a) clinoptilolite in the dark (34% reduction at 120 hours), (b) montmorillonite in the dark (26% reduction at 120 hours), and (c) empty beads in the dark (20% reduction at 120 hours). The percent reductions after 120 hours in the presence of light were smaller: (a) 13% for clinoptilolite, (b) 6% for montmorillonite, and (c) 16% for empty beads. The signal intensity in flasks containing montmorillonite in the presence of light increased after 1, 4, and 24 hours of incubation (Figure 7).

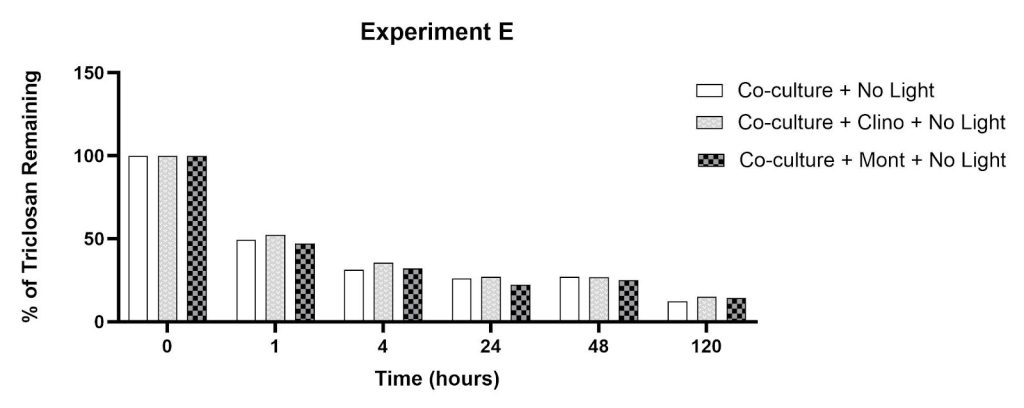
The amount of triclosan remaining after incubation with co-culture microalgae beads and individual clay minerals in complete darkness is shown in Figure 8. The steepest reductions in triclosan were observed within 1-4 hours (48%-69%), with steady reductions continuing through 120 hours. There were minimal differences in this trend regardless of the presence of clinoptilolite or montmorillonite. By t=120 hours, the percent reductions in the co-culture flasks with montmorillonite, clinoptilolite, and no-clay were comparable (86%, 85%, 88% for co-culture/mont, co-culture/clino, co-culture alone, respectively).
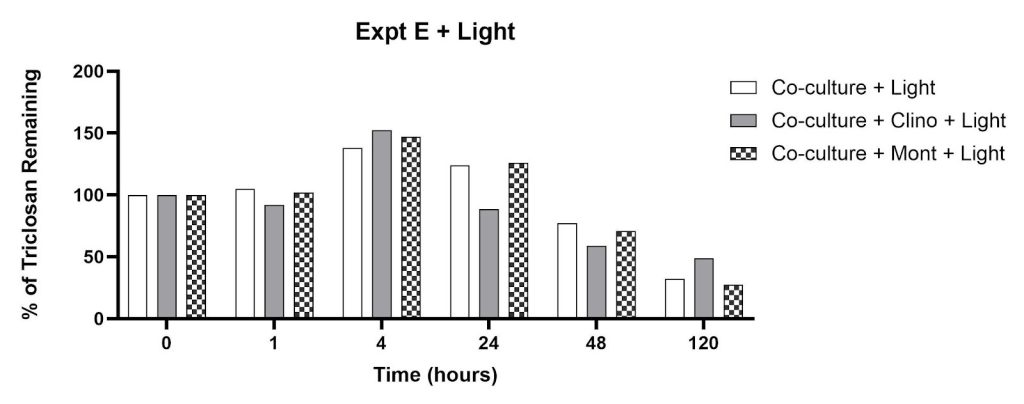
In contrast, flasks exposed to 12 hours of light per 24-hour period showed increases in signal intensity at 280 nm after 4 hours of incubation, which was sustained through 24 hours before decreasing between 48 and 120 hours (Figure 9). By t=120 hours, the percent triclosan reductions from the co-culture flasks with montmorillonite, clinoptilolite, and no-clay varied moderately (72%, 64%, 68% for co-culture/mont, co-culture/clino, co-culture alone, respectively).
Discussion
Restatement of Key Findings
Data from Experiments A and B showed the impact of immobilized microalgae (C. pyrenoidosa, S. quadricauda) on triclosan removal, with noteworthy triclosan reductions after 4-6 hours compared to empty beads under specific experimental conditions. The results demonstrate that the microalgae was responsible for remediating the triclosan, rather than the polymer casing of the bead itself. The rapid reduction of triclosan by microalgae beads (either mono- or co-culture) confirmed our hypothesis that immobilized beads might be viable remediators. The quick action suggests bioadsorption as the removal mechanism, although researchers report rapid biodegradation of triclosan by planktonic C. pyrenoidosa20.
In Experiment B, it was unexpected to observe increases in HPLC signal intensity during 4-24 hours in the presence of microalgae beads (Figure 5), clay minerals (Figure 7), and microalgae/clay combinations (Figure 9) exposed to light. We would expect that all samples exposed to light would have less triclosan present over time, due to the half-life of triclosan in aqueous solution and photodegradation products characterized in the literature30. Researchers have documented the release of proteins and other compounds by microalgae exposed to high doses of triclosan, which might inflate the signal intensity at 280 nm and apparent concentration of triclosan20. However, this speculation does not account for the increase in signal intensities for the clay-only flasks. It is possible that photoinduced dimerization of triclosan might be taking place at these concentrations, which would lead to an increase in signal intensity due to the higher molar absorptivity of triclosan dimers31. Future work with HPLC-MS is needed to confirm the nature of the increases in peak area.
Implications and Significance
These preliminary findings might have implications for municipal wastewater processing. The alginate beads offer the chance to be integrated into existing processing facilities because they can be handled more easily than planktonic cultures of C. pyrenoidosa and S. quadricauda. After sufficient remediation, the microalgae could be harvested from the beads, further processed, and used as biofeedstocks for other products. The observed removal results from cultures incubated in the dark also suggest that access to light during processing may not be required.
Connection to Objectives
Measuring triclosan reductions during incubation with microalgae beads and clay minerals were the 2 objectives achieved in this study. We are pleased that reductions of 64-88% were achieved in 4-6 hours with microalgae beads, which is similar to the results obtained when planktonic microalgae is used. We observed that supplementing microalgae with clay minerals does not significantly boost triclosan removal percentages at neutral pH and can conclude that clay minerals would not be a useful supplement to improve upon the 64-88% reductions.
Recommendations
Several recommendations are offered for future studies. First, the possible mechanisms of triclosan removal should be addressed by using HPLC-MS to characterize the composition of the peaks at retention times consistent with triclosan. Second, head-to-head comparisons of immobilized forms of C. pyrenoidosa, S. quadricauda and the co-cultures of these strains might uncover which strain is the faster remediator or employs a more efficient removal mechanism. Furthermore, characterizing the bioaccumulation vs. biodegradation pathways of triclosan removal may inspire the use of different algae consortia to improve speed and efficiency of triclosan remediation.
Limitations
The percent decreases in triclosan concentrations for select experimental groups are valid on a preliminary basis. However, mass spectrometry (MS) coupled with HPLC is needed to confirm the composition of the peaks in spite of their symmetry and consistent retention times. The nature of these heterogeneous samples (microalgae, alginate bead, clay minerals) requires additional analysis.
Closing Thought
This study is likely the first to explore how immobilized co-cultures of C. pyrenoidosa and S. quadricauda might remove triclosan from wastewater. Our findings suggest that these strains in alginate bead form can reduce triclosan concentrations after short incubation periods in the dark. Moreover, once the microalgae has remediated the target pollutants, these cells could be harvested for biofuel or biofeedstock. Further work is needed to optimize how microalgae beads (mono-culture, co-culture, strain adaptability) can remove antibiotics accumulating in our environment after they have served their purpose.
Acknowledgements
We would like to thank the Science Department of Flintridge Sacred Heart Academy (La Canada, CA), as well as Dr. Marc Baum and Amalia Castonguay of the Oak Crest Institute of Science (Monrovia, CA) for their support of our scientific activities. We would also like to thank the editors of The National High School Journal of Science for their review and publication of this paper.
References
- X. Qiao, X. Zheng, Q. Xie, X. Yang, J. Xiao, W. Xue, J. Chen. Faster photodegradation and higher dioxin yield of triclosan induced by CTAB. Journal of Hazardous Materials. 275, 210–214 (2014). [↩] [↩] [↩] [↩] [↩]
- J.N. Apell, S. Kliegman, C. Solá-Gutiérrez, K. McNeill. Linking triclosan’s structural features to its environmental fate and photoproducts. Environmental Science & Technology. 54, 14432–14441 (2020). [↩] [↩] [↩]
- J. Mo, L. Han, R. Lv, M.W. Chiang, R. Fan, J. Guo. Triclosan toxicity in the cyanobacterium Anabaena flos-aquae: growth, photosynthesis and transcriptomic response. Journal of Environmental Sciences. 127, 82–90 (2022). [↩]
- M.F. Yueh, R.H. Tukey. Triclosan: a widespread environmental toxicant with many biological effects. Annual Review of Pharmacology and Toxicology. 56, 251–272 (2016). [↩]
- J. M. Buth, P. O. Steen, C. Sueper, D. Blumentritt, P. J. Vikesland, W. A. Arnold, K. McNeill. Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environmental Science & Technology. 44, 4545–4551 (2010). [↩]
- N. A. Jayalatha, C. P. Devatha. Degradation of triclosan from domestic wastewater by biosurfactant produced from Bacillus licheniformis. Molecular Biotechnology. 61, 674–680 (2019). [↩] [↩]
- J. Q. Xiong, M. B. Kurade, B. H. Jeon. Can microalgae remove pharmaceutical contaminants from water? Trends in Biotechnology. 36, 30–44 (2018). [↩] [↩]
- V. Kuokkanen, T. Kuokkanen, J. Rämö, U. Lassi. Recent applications of electrocoagulation in water and wastewater treatment—a review. Green and Sustainable Chemistry. 3, 89–121 (2013). [↩] [↩]
- Beyond Pesticides. Proctor and Gamble to eliminate triclosan from its products by 2014. Beyond Pesticides Daily News Blog. (2013). Available at: https://beyondpesticides.org/dailynewsblog/2013/09/proctor-and-gamble-to-eliminate-triclosan-from-its-products-by-2014/. [↩]
- U.S. Food & Drug Administration. Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use; proposed amendment of the tentative final monograph; reopening of administrative record. Federal Register. 78, 76444–76479 (2013). [↩]
- A. Kodjak. FDA bans 19 chemicals used in antibacterial soaps. NPR Health Shots. (2016). Available at: https://www.npr.org/sections/health-shots/2016/09/02/492394717/fda-bans-19-chemicals-used-in-antibacterial-soaps. [↩]
- European Commission. Commission Implementing Decision (EU) 2016/110 of 27 January 2016 not approving triclosan as an existing active substance for use in biocidal products for product-type 1. Official Journal of the European Union. (2016). Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2016.021.01.0086.01.ENG&toc=OJ:L:2016:021:FULL. [↩]
- A. Rempel, J. P. Gutkoski, M. T. Nazari, G. N. Biolchi, V. A. F. Cavanhi, H. Treichel, L. M. Colla. Microalgae-based bioremediation and other technologies for emerging contaminants. Science of the Total Environment. 772, 144918 (2021). [↩] [↩]
- R. Kothari, A. Pandey, S. Ahmad, H. M. Singh, V. V. Pathak, V. V. Tyagi, K. Kumar, A. Sari. Utilization of Chlorella pyrenoidosa for remediation of common effluent treatment plant wastewater in coupling with co-relational study. Bulletin of Environmental Contamination and Toxicology. 108, 507–517 (2021). [↩] [↩]
- A. Solé, V. Matamoros. Removal of endocrine-disrupting compounds from wastewater by microalgae co-immobilized in alginate beads. Chemosphere. 164, 516–523 (2016). [↩]
- X. Bai, K. Acharya. Algae-mediated removal of selected pharmaceutical and personal care products (PPCPs) from Lake Mead water. Science of the Total Environment. 581–582, 734–740 (2017). [↩]
- Q. Guo, E. R. Bandala, A. Goonetilleke, N. Hong, Y. Li, A. Liu. Application of Chlorella pyrenoidosa-embedded biochar beads for water treatment. Journal of Water Process Engineering. 40, 101892 (2021). [↩]
- Z. Xie, X. Wang, Y. Gan, H. Cheng, S. Fan, X. Li, J. Tang. Ecotoxicological effects of fluoxetine and its removal by Chlorella pyrenoidosa. Ecotoxicology and Environmental Safety. 244, 114045 (2022). [↩]
- S. Wang, K. Poon, Z. Cai. Removal and metabolism of triclosan by three microalgal species. Journal of Hazardous Materials. 342, 643–650 (2018). [↩] [↩] [↩] [↩] [↩] [↩]
- S. Wang, X. Wang, K. Poon, Y. Wang, S. Li, H. Liu, S. Lin, Z. Cai. Removal and reductive dechlorination of triclosan by Chlorella pyrenoidosa. Chemosphere. 92, 1498–1505 (2013). [↩] [↩] [↩] [↩] [↩]
- T. I. Oliomogbe, J. O. Emegha. Application of Scenedesmus quadricauda biosorbent for heavy-metal removal in wastewater. Science and Technology Asia. 29, 213–223 (2024). [↩]
- F. Bano, A. Malik, S. Z. Ahammad. Removal of estradiol, diclofenac and triclosan by naturally occurring microalgal consortium obtained from wastewater. Sustainability. 13, 7690 (2021). [↩] [↩]
- P. Rajasulochana, V. Preethy. Sewage treatment using green algae Scenedesmus, Chlorella and their combination. Desalination and Water Treatment. 149, 76–90 (2019). [↩]
- K. Styszko, K. Nosek, M. Motak, K. Bester. Clay minerals for removal of pharmaceuticals, bisphenol A and triclosan. Comptes Rendus Chimie. 18, 1134–1142 (2015). [↩] [↩]
- S. K. Behera, S. Y. Oh, H. S. Park. Sorption of triclosan onto activated carbon, kaolinite and montmorillonite: effects of pH, ionic strength and humic acid. Journal of Hazardous Materials. 179, 684–691 (2010). [↩]
- T. A. Kurniawan, M. H. D. Othman, M. R. Adam, X. Liang, H. Goh, A. Anouzla, M. Sillanpää, A. Mohyuddin, K. W. Chew. Chromium removal from aqueous solution using natural clinoptilolite. Water. 15, 1667 (2023). [↩]
- B. D. S. G. Proença, R. de Souza Antonio, L. F. Cusioli, M. F. Vieira, R. Bergamasco, A. M. S. Vieira. Adsorptive removal of triclosan by natural zeolite. Desalination and Water Treatment. 292, 165–175 (2023). [↩]
- H. H. Murray. Traditional and new applications for kaolin, smectite and palygorskite: a general overview. Applied Clay Science. 17, 207–221 (2000). [↩]
- F. Uddin. Montmorillonite: an introduction to properties and utilization. In Montmorillonite. 3–23 (IntechOpen, London, 2018). [↩]
- X. Qiao, X. Zheng, Q. Xie, X. Yang, J. Xiao, W. Xue, J. Chen. Faster photodegradation and higher dioxin yield of triclosan induced by CTAB. Journal of Hazardous Materials 275, 210–214 (2014). [↩]
- P. Wong-Wah-Chung, S. Rafqah, G. Voyard, M. Sarakha. Photochemical behaviour of triclosan in aqueous solutions: kinetic and analytical studies. Journal of Photochemistry and Photobiology A: Chemistry. 191, 201–208 (2007). [↩]