Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs) are known for their immunomodulatory and anti-inflammatory effects that makes them promising candidates for treating patients with COVID-19 and COPD. At this point in time, effective treatment options for these two respiratory diseases remain limited. Several studies that investigated UC-MSC therapy have been presented with capable results in controlling inflammation. Clinical trials presented improvements in many aspects of COVID-19, including reduction in inflammatory cytokines, improved oxygenation, and enhanced lung tissue repair. While limited studies have been conducted on COPD, early results point to a successful direction, such as improving symptoms like dyspnea and reducing exacerbations. A number of alternative methods have also been investigated for COPD and COVID-19 such as antiviral therapies and systemic corticoids, but they didn’t prove to be as effective. Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs) and Adipose Tissue-Derived Mesenchymal Stem Cells (AT-MSCs) are also types of mesenchymal stem cells that have been looked into as a therapeutic approach to manage inflammatory symptoms, but the advantages of UC-MSCs outweigh these options. Through phase I and phase II trials, UC-MSC treatment has been concluded to be safe and efficacious to an extent.
Introduction
Coronavirus disease 2019 (COVID-19) and Chronic Obstructive Pulmonary Disease (COPD) are both respiratory diseases that damage the lungs. COVID-19 has caused over 7 million deaths worldwide and COPD remains the third leading cause of death, causing 3.23 million deaths in 2019. One of the main pathological features in COVID-19 and COPD is inflammation. COVID-19 and COPD share many molecular pathways involved in inflammation and immune response. This overlap hints at the possibility to use similar treatment approaches for both conditions.
COVID-19, which is caused by the novel coronavirus SARS-CoV-2, impacted the world population with currently over 700 million cases reported as of August 2024, ever since its outbreak in late 2019. COVID-19 leads to human respiratory tract infections, which can range from mild colds to severe respiratory distress syndromes1. Severe cases of COVID are often associated with a hyperinflammatory state involving the excessive release of a storm of cytokines (“cytokine storm”) that over-activates the immune response to COVID-192.
Some of the many treatment options for COVID-19 include antiviral and anti-inflammatory therapies combined. Unfortunately, studies have shown that an anti-inflammatory treatment strategy was not effective in overcoming and decreasing mortality. In hospitalized adults with severe COVID-19, lopinavir-ritonavir treatment, an antiviral therapy, was added to standard care and was seen to not be associated with clinical improvement, mortality, or duration of a hospital stay3. Corticoids were also used to see if there would be improvement. A common systemic corticoid, dexamethasone, is frequently used to decrease systemic inflammation and was provided to patients as a treatment option. Nevertheless, the World Health Organization (WHO) does not recommend the use of corticoids as they inhibit the immune response, which is crucial for protecting the host against viruses and could end up being harmful to the patient. This means that it could lead to an increased likelihood of developing secondary infections4. A study that examined 1540 patients with severe COVID-19 found that 221 developed hospital-acquired infections (HAI), where they used corticosteroids as a treatment. Treatment with corticosteroids was associated with the development of HAI5. However, the study did find that corticosteroids could reduce mortality in severe COVID-19 patients, but was not effective in non-severe patients and was related to a longer hospital stay. This leaves the treatment efficacy mostly inconclusive. Since these options seem insufficient, researchers are looking into UC-MSCs for treatment as they might be safe, effective and accessible. UC-MSCs have gained significant attention in recent years due to several key advantages. They can be collected through a non-invasive procedure, avoiding any ethical concerns tied to embryonic stem cells or invasive adult stem cell collection. Embryonic stem cells are derived from early-stage embryos, which raise ethical concerns because their collection involves destroying the embryo. On the other hand, adult stem cells are taken from adult tissues, but collecting them requires invasive procedures such as surgeries or biopsies. UC-MSCs possess immunoprivileged status, meaning they express low levels of major histocompatibility complex (MHC) molecules, reducing the risk of immune rejection after transplantation. Additionally, UC-MSCs exhibit strong immunomodulatory effects such as suppressing overactive immune responses and reducing inflammation through secreting anti-inflammatory cytokines and growth factors. This quality of repair and remodeling of damaged lung tissue makes them particularly attractive for respiratory diseases characterized by inflammation and tissue injury.
Chronic Obstructive Pulmonary Disease (COPD) is a respiratory condition that narrows the airways, making it difficult to breathe. In a modeling study, COPD prevalence is to approach 600 million cases worldwide by 20506. That means that around every 1 in 13 people would have COPD. COPD is defined by three factors: Chronic bronchitis, which is the inflammation of the airways7. Macrophage bronchiolitis, which is generally dependent on the accumulation of T-cells and B-lymphocytes in the airways7. And emphysema, which destroys the alveolar wall7. Furthermore, COPD could impose serious health risks that include cardiovascular risk, respiratory failure, and lung cancer6. COPD patients exhibit chronic inflammation induced by the release of inflammatory mediators. However, the inflammation seen in COPD has not been associated with cytokine storms.
Current, available treatments for COPD include pharmacological and non-pharmacological approaches, but they still remain inefficient. Inhaled medicines like long-acting bronchodilators and ICS/LABA combos help with symptoms but can cause side effects for some patients8. Doctors use systemic corticosteroids and antibiotics during exacerbations. But these can make infections more likely. Non-drug treatments, like ventilatory support and pulmonary rehabilitation, are crucial but can hold challenges since it is not a long term solution. The risk of spreading infection also increases so ventilatory support is not considered an ideal treatment option for chronic conditions like COPD8. These shortcomings show we need COPD treatments that work better and are safer. UC-MSCs can be a potential pathway for COPD treatment because it addresses these shortcomings with their potent ability to modulate the immune environment by shifting the balance from pro-inflammatory to anti-inflammatory mediators. They secrete bioactive molecules such as prostaglandin E2, transforming growth factor-beta (TGF-β), and interleukin-10 (IL-10), which help resolve chronic inflammation and promote tissue repair9,10. UC-MSCs are also very easy to access as they are derived from discarded umbilical cord tissue. Compared to other stem cell types, UC-MSCs raise fewer ethical concerns since their collection does not harm the donor or involve the destruction of embryos. This makes them more acceptable for use in the medicine.
The inflammatory condition in both of these diseases has shown areas for a solution in regenerative medicine through Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSC) research. UC-MSCs, which are isolated from gelatinous tissue around umbilical vessels known as Wharton’s Jelly, have the ability to communicate with immune cells—involving cell-to-cell contact—by releasing factors11. The role of inflammation in both COVID-19 and COPD and, the rationale for using UC-MSC therapies to treat COVID-19 and COPD will be examined and the clinical efficacies compared between the two diseases.
Research Question
How safe are UC-MSCs in treating inflammation in COVID-19 and COPD, and what is their potential efficacy as a therapeutic option?
Methods
By using published studies from databases like PubMed and ClinicalTrails.gov, specific studies have been included based on certain criteria.
ClinicalTrails.gov was the database used to find clinical trials of UC-MSCS on either COPD and COVID-19. Inclusion criteria were defined to include peer-reviewed, human clinical trials published between January 2020 and December 2024. These studies were all investigating the use of UC-MSCs for treating inflammation in either of the diseases. Eligible studies included randomized controlled trials, non-randomized clinical trials, and pilot studies that reported outcomes focused on the safety, tolerability and efficacy of the treatment. Exclusion criteria includes uncompleted trials, in vitro studies, animal trials and studies that were not published in the English language. Only completed trials with posted results were included in the paper, with the exception of one singular study protocol in the COPD trials section. For Clinicaltrials.gov search, results from the search for “COVID-19” or “COPD” as the disease and “UC-MSCs” as the intervention/treatment were identified and considered for screening.
Trials from ClinicalTrials.gov that had completed results posted on PubMed were used for this research paper. Additionally, any papers that are relevant on this topic were also included in this review. Papers on the topic of inflammation, cytokine storms, therapeutic approaches listed in this review, COPD, COVID-19, etc. were used as supporting information. For PubMed search, key phrases include, “UC-MSCs COVID-19 clinical trial”, “UC-MSCs COPD efficacy”, “COVID-19 and UC-MSCs” and “mesenchymal stem cells inflammation”. Only full free available texts were included in the paper.
Figure 1. Study Selection Process using ClinicalTrials.gov for COVID-19 and COPD.
Figure 1 outlines the study selection process. Clinicaltrials.gov presented a total of 32 with the mentioned search inputs. Search results on Clinicaltrails.gov for diseases as “COVID-19” and the intervention/treatment as “UC-MSCs” ended up presenting 28 studies: 2 were not recruiting, 8 were recruiting, 6 were completed, 3 were withdrawn, and 9 has an unknown status. Out of these, 6 clinical trials with posted results (on PubMed) were included in the clinical trials section of the review. Search results on Clinicaltrials.gov for “COPD” as the disease and “UC-MSCs” as the intervention/treatment, ended up presenting 4 studies: 2 were recruiting and 2 were completed. The 2 completed trials with posted results (on PubMed) were included in the clinical trials section of the review.
Inflammation
Inflammation in COVID-19
Patients with COVID-19 have been seen with higher levels of pro-inflammatory cytokines including IL-1, IL-2, IL-6, TNF-α, IFN-γ, IP-10, GM-CSF, and MCP-12. Cytokines are polypeptides that play a role in pathophysiological processes such as inflammation, tissue repair, fibrosis and coagulation. While they seem important in the immune system, cytokines can actually become harmful to the human body when they are produced excessively, ultimately leading to a cytokine storm2. Typically, the production for pro-inflammatory and anti-inflammatory cytokines are balanced, maintaining equilibrium12. However, it can be interfered with by factors such as infections, chronic diseases, autoimmune diseases, the use of medications and others that disturb the balance. These, in turn, activate immune cells like neutrophils, macrophages, Natural Killer cells, and T-cells. Consequently, this increases the production of more pro-inflammatory cytokines2,13. Macrophages, when activated, produce pro-inflammatory cytokines that contribute to the inflammatory response. NK cells contribute to the cytokine storm through impaired function or excessive amounts of IL-62. Natural Killer cells are usually responsible for killing infected or cancerous cells. If they are impaired, there can be chronic antigenic stimulation or may fail to respond to signals2. Because of the activation of the immune cells more inflammatory mediators and immune cells are needed at the site. This positive feedback loop in turn increases the response. Thus, worsening the condition.
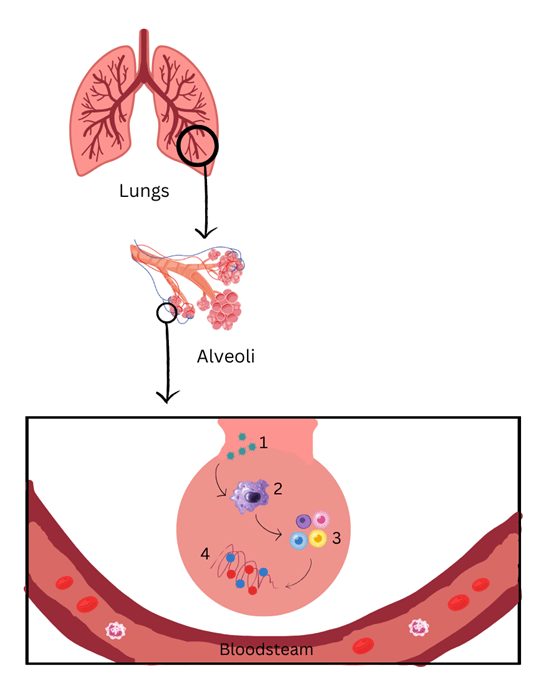
Figure 2. Immune Cells in Cytokine Storms Figure 2 represents the role that immune cells play in cytokine storms that progress towards lung damage. As it enters, SARS-CoV-2 virus (1) infects the lungs cells. Immune cells (2), including macrophages, start to produce pro-inflammatory cytokines (3). The secretion of pro-inflammatory cytokines eventually causes a cytokine storm (4). Created on Canva.com
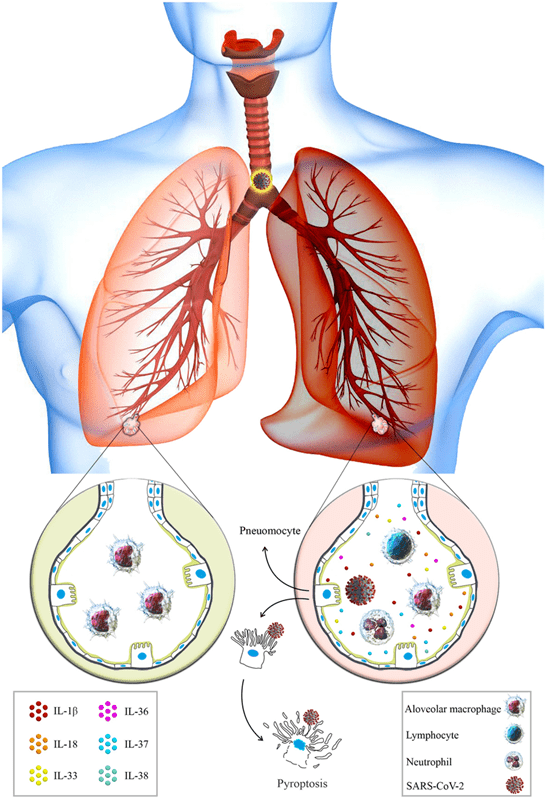
Figure 3. Healthy Alveoli VS Damaged Alveoli in SARS-CoV-214 Figure 3 is another visual comparing a healthy alveoli (on the left) to a damaged alveoli (on the right). The different immune cells, such as lymphocytes and neutrophils, can be noticed in the damaged alveoli, along with the SARS-CoV-2 infection. These immune cells are not present in the alveoli under normal conditions. Cytokine production recruits more immune cells to the site, triggering the inflammatory response in the lungs. Adapted from Azvudine versus Paxlovid in COVID-19: A systematic review and meta-analysis (2024).
Inflammation in COPD
Generally, the inflammatory cells that enter the lung’s airways are what causes the chronic inflammation in COPD. These inflammatory cells are often induced by triggers like cigarette smoke or environmental pollutants. Inflammatory cells refer to neutrophils, macrophages, lymphocytes, and eosinophils. In a patient with COPD, neutrophils are recruited to the airways of the lungs. Proteases such as neutrophil elastase (NE), matrix metalloproteinase (MMP) and myeloperoxidase (MPO) help in the destruction of alveoli, small air-sacs inside the lungs that perform gas exchange15. This relates to the third factor, emphysema. Macrophages are leukocyte-derived inflammatory cells that are mononuclear. Since they also release inflammatory mediators and enzymes it amplifies the inflammatory response. This would end up in the obvious contribution to inflammation in COPD patients.
Furthermore, smoking causes more macrophages to be present in bronchial tissue7. It has been reported that there is an increased number of macrophages in the airways of smokers compared to non-smoker7. This is because when cigarette smoke comes in contact with lung epithelial cells and alveolar macrophages, it triggers the release of cytokines like IL-8, TNF-α, and interleukin-1β7. These cytokines are main contributors to inflammation in patients. Lymphocytes, which include T-cells and B-cells, accumulate in the airways of the lungs. This process is macrophage bronchiolitis, the second factor of COPD. Lymphocytes, particularly CD8+ and CD4+ cells, play a significant role in the induction and progression of COPD. Their count and activity at the level of the airways and blood are associated with the severity and progression of the disease15,7. While eosinophils are less prominent, they can be related to a specific subtype of COPD. For the most part, eosinophilic inflammation is more commonly associated with asthma. This therefore indicates that some COPD patients have asthma-like features, including the involvement of eosinophils in the disease7. The combined action of these inflammatory cells finally results in a continuous cycle of inflammation, tissue destruction, and airway remodeling—all processes contributing to the progressive nature of COPD.
Potential of UC-MSCs for inflammation
UC-MSC therapies stand out from the many other methods to treat inflammation in COVID-19 and COPD because of the various things they have to offer. First, it’s their ability to change the immune system response, known as their immunomodulatory properties, and reduce inflammation, which are their anti-inflammatory properties16. UC-MSCs can prevent the body from producing too many pro-inflammatory cytokines, which are responsible for worsening the inflammatory state9.
At the same time, they boost anti-inflammatory cytokines like IL-109,10. T-cells are a significant part of the immune system. They benefit the body by protecting it from infections and diseases and attack the infected cells. There are different types of T-cells with various functions, one of them being effector T-cells. Effector T-cells help with the activation of T-cells that attack. Regulatory T-cells, another type, help control and regulate the immune response. In terms of inflammation, they help prevent T-cells from becoming dangerous and attacking healthy cells. Effector T-cell inhibition is also a key effect of UC-MSCs16. They induce T-cell apoptosis, a type of programmed cell death (PCD) by secreting indoleamine 2, 3-dioxygenase (IDO), prostaglandin E2 (PGE-2), and transforming growth factor-β1 (TGF-β1)11. In addition to reducing the frequency of effector T-cells, UC-MSCs increase the number of regulatory T-cells to help maintain the immune system’s inflammatory response through secreting indoleamine 2, 3-dioxygenase (IDO), prostaglandin E2 (PGE-2), and transforming growth factor-β1 (TGF-β1)11. UC-MSCs also prevent dendritic cells from maturing. By capturing and presenting antigens to T-cells, dendritic cells activate T-cells, causing an immune response11. So by preventing dendritic cell maturation, they can’t activate T-cells, suppressing inflammation. In addition to dendritic cells, UC-MSCs change monocytes to produce anti-inflammatory substances, turning it into an IL-10-producing phenotype11,10. By secreting factors like IL-6 and HGF, UC-MSCs change the monocyte. UC-MSCs inhibiting B-cells from proliferating, differentiating and producing antibodies have been noticed11.
However, this effect is not well-researched yet. Unlike Adipose Tissue-Derived Mesenchymal Stem Cells (AT-MSC) and Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSC), UC-MSCs exhibit lower expression of the gene major histocompatibility complex I (MHC I) and no expression of the gene MHC II17. This would reduce the likelihood of immune rejection. In fact, UC-MSCs have proven to be a better choice for treatment compared to AT-MSCs and BM-MSCs. UC-MSCs have “lower immunogenicity, greater proliferation and differentiation potential, a slower senescence rate, and greater anti-inflammatory and immunomodulatory effects” than AT-MSCs and BM-MSCs, proving itself as a better candidate for pulmonary diseases. While both UC-MSCs and BM-MSCs possess immunomodulatory effects, a study has shown that UC-MSCs have superior migration abilities toward activated lymphocytes compared to BM-MSCs18. In response to chemokines, UC-MSCs showed higher migration in response to chemokines. Beyond this, the immunomodulatory and regenerative properties of BM-MSCs can vary based on multiple factors: donor age, health status, and culture conditions19. The variability makes it not only harder to investigate, but also to retrieve BM-MSCs. This also explains why there is a lack of clinical trials with the use of BM-MSCs and respiratory conditions such as COPD and COVID-19. However, with further trials of BM-MSCs, an accurate conclusion can be made about their potential efficacy. A unique benefit that applies to UC-MSCs only, is that they are “considered a waste product after birth”17. This makes them easier to obtain and it also implies that there are no ethical concerns behind the research.
Table 1. Comparison of MSCs: UC-MSCs, AT-MSCs, BM-MSCs. The table compares the mesenchymal stem cells derived from three different sources: umbilical cord, adipose tissue and bone marrow. The table compares their different properties. As it can be noticed, UC-MSCs have shown greater proliferation rate, differentiation potential, anti-inflammatory properties and immunomodulatory effects which explains their increasing use in research.
| Property | UC-MSCs | AT-MSCs | BM-MSCs |
| Source | Umbilical cord | Adipose tissue | Bone marrow |
| Immunogenicity | Low (MHC I, no MHC II) | Moderate (MHC I, low MHC II) | Moderate (MHC I, low MHC II) |
| Proliferation Rate | High | Moderate | Low |
| Differentiation Potential | High | Moderate | Moderate |
| Anti-inflammatory properties | Strong | Moderate | Moderate |
| Immunomodulatory Effects | Strong | Moderate | Moderate |
| Ethical Concerns | None (waste product after birth) | Low (invasive extraction) | Moderate (invasive extraction) |
| Availability | High | Moderate | Limited |
Clinical Trials
Clinical trials are essential for determining safety and efficiency for any pulmonary disease, including COVID-19 and COPD. There are different types of clinical trials: phase 0 (pilot), phase I, phase II, phase III and phase IV. Phase 0, also known as a pilot phase, is done in human volunteers and is conducted to see how well the body tolerates the treatment. Phase 0 clinical trials are done in small doses. Phase I is for a slightly larger group and primarily focuses on safety of the treatment. Phase II assesses the drug’s efficacy in a small group of patients while phase III determines efficacy on a large scale. Phase IV tracks safety and effectiveness in the general population after the treatment is approved for use. Studies in this paper include phase 0, I and II as UC-MSC therapy remains an emerging area of treatment. In the following sections, 6 clinical trials for COVID-19 and 2 clinical trials for COPD will be presented with a focus for safety while also discussing some indications of efficacy.
Use of UC-MSCs in COVID-19
Severe cases of COVID-19 are usually characterized with a rise in pro-inflammatory cytokine and chemokine levels. This, consistently, leads to respiratory and cardiovascular failure, organ damage and even worse, death. A handful of pulmonary diseases damage the lungs through an unregulated immune system, like COVID-19. But the solution lies ahead in UC-MSCs as they have been found with immunomodulatory effects that can reverse the immune response. This holds a lot of potential for treatment if researched thoroughly. After reviewing clinical trials with posted results on Clinicaltrials.gov, 6 completed studies were included for COVID-19.
Table 2. Completed UC-MSC Clinical Trials for COVID-19. This table shows different study designs that used UC-MSC therapy as a potential treatment option for COVID-19. Key features include study design, sample size, cell dosage, primary and secondary outcomes, follow-up duration, and statistical significance of reported findings. P-values below 0.05 were considered statistically significant. The trials were different from each other but they mostly assessed factors like safety, inflammatory markers (e.g., IL-6), and clinical outcomes such as mortality and oxygenation.
| References | Study Design | Sample Size: Experimental Group | Sample Size: Control Group | Dosage | Primary and Secondary outcomes | Follow-up duration | Statistical significance P-values less than 0.05 are considered statistically significant |
| Meng et al. (2020)20 | Non-randomized, phase I clinical trial | 9 | 9 | 3 cycles of 3 × 10⁷ cells/infusion. | Primary: safety (adverse events)-no adverse events observed Secondary: Mechanical Ventilation Low-flow oxygen support High-flow oxygen support Fever Fatigue Cough Shortness of Breath IgM antibody IgG antibody Interval between admission and charge | Patients were followed up throughout their hospital stay. Serial measurements on days 0, 3, 7 and cytokine levels measured up to day 14. Average Hospital Stay: UC-MSCs group: 20 days Control: 23 days | Mechanical Ventilation: 0.294 Low-flow oxygen support: 1.000 High-flow oxygen support: 0.637 Fever: 0.335 Fatigue: 1.000 Cough: 0.131 Shortness of Breath: 0.131 IgM antibody: 0.114 IgG antibody: 0.174 Interval between admission and charge: 0.306 |
| Hashemian et al. (2021)21 | Non-randomized, Phase I clinical trial | 11 | 0 | 600 × 10⁶ cells | Primary: safety (adverse events)- no serious adverse events reported for 9 out 11 patients. 2 patients experienced mild shivering after PL-MSC infusion which resolved within an hour. Secondary: Respiratory improvement (ex. respiratory rate, dyspnea relief, etc.) Cytokine level changes Mortality and ICU discharge timing Lymphocyte and WBC profiles | Follow-up period: 60 days | SpO₂ improvement: p = 0.01 Reduction in TNF-α: p < 0.01 Reduction in IL-8: p = 0.02 Reduction in CRP: p < 0.01 WBC differences between survivors and non-survivors: p = 0.01 Lymphocyte % <10% associated with mortality: p < 0.01 |
| Lanzoni et al. (2021)22 | Randomized controlled trial, phase I | 12 | 12 | 20 × 10⁶ cells | Primary: safety (adverse events)- (a) the occurrence of prespecified infusion-associated adverse events (AEs) within 6 hours from each infusion; (b) cardiac arrest or death within 24 hours postinfusion; and (c) incidence of AEs. Serious adverse events (SAEs): 2 in the UC-MSC group; 16 in the control group (P = 0.04) Secondary: Survival SAE-free survival Time to recovery Inflammatory markers | 31 days after first infusion, which equals 28 days after second infusion | Survival: 0.015 SAE-free survival: 0.008 Time to recovery: 0.031 Inflammatory markers: p < 0.05 for most markers-significant reductions in 9 out of 10 tested markers for UC-MSC group only |
| Shi et al. (2020)23 | Randomized, phase II clinical trial | 65 | 35 | 3 cycles of 4 × 10⁷ cells/infusion | Primary: Change in the total lesion proportion (%) of the whole lung volume from baseline to day 28, measured by high-resolution chest CT Secondary: Change in solid component lesion volume (more fibrotic-type lung lesions) 6-Minute Walk Test (6-MWT) at Day 28 Oxygen therapy duration | The follow-up duration was 28 days after randomization. All patients were assessed at Day 10 and Day 28 post-treatment. | Total lesion volume change (primary): 0.080 Solid component lesion reduction: 0.043 6-minute walk test improvement: 0.057 Duration of oxygen therapy: ~0.09 mMRC dyspnea score: not significant Cytokines and immune cell subsets: not significant Adverse events (safety): not statistically significant |
| Shu et al. (2020)24 | Randomized controlled trial, phase I/II | 12 | 29 | 2 × 10⁶ cells/kg (patient body weight) | Primary: Progression from severe to critical illness 28-day mortality rate Time to clinical improvement (defined as a 2-point drop on a 7-point ordinal scale) Secondary: Clinical status at Days 3, 7, 14, and 28 Hospital stay duration Changes in inflammatory markers (CRP, IL-6) Lymphocyte recovery Oxygenation index CT imaging improvements | Follow-up was for 28 days after treatment Patients were monitored at multiple time points: Day 3, Day 7, Day 14, and Day 28 | Time to clinical improvement (overall): 0.006 Time to improvement (age ≤ 65): 0.0014 Time to improvement (age > 65): < 0.001 Clinical improvement by Day 7: 0.020 Clinical improvement by Day 14: 0.030 CT score (2 weeks): 0.017 Consolidation: 0.0306 Day 28 mortality: 0.543 Progression to critical illness: 0.667 Hospital stay: 0.054 |
| Dilogo et al. (2021)25 | Randomized controlled trial, phase II | 20 | 20 | 1 × 10⁶ cells/kg (patient body weight) | Primary: Mortality rate Length of ventilator usage Secondary: Length of stay in the ICU Improvement in routine lab values: CBC, CRP, D-dimer, fibrinogen, procalcitonin Improvement in biomarkers: IL-6, IL-10, VEGF, LIF, Ferritin Lymphocyte subpopulations: CD4-CXCR3, CD8-CXCR3, CD56-CXCR3 Adverse events (AEs) or serious adverse events (SAEs) | Patients were monitored for 15 days post-intervention Cytokine and immune markers were specifically assessed on Day 0 and Day 7 | Mortality rate difference: 0.047 Survival rate among patients with ≥2 comorbidities: 0.023 IL-6 reduction in recovered patients: 0.023 LIF increase in MSC group: 0.002 Length of ventilator use: not significant Length of ICU stay: not significant Complete blood count (CBC) values (Hb, Hct, WBC, etc.): not significant CRP and procalcitonin: not significant D-dimer and fibrinogen: not significant IL-10 levels: 0.661 (not significant) VEGF: 0.826 (not significant) Ferritin: 0.861 (not significant) CD8-CXCR3 and CD56-CXCR3 suppression: 0.061 (not significant) CD4-CXCR3: 0.064 baseline, 0.745 post-treatment (not significant) No significant AEs or SAEs observed in either group |
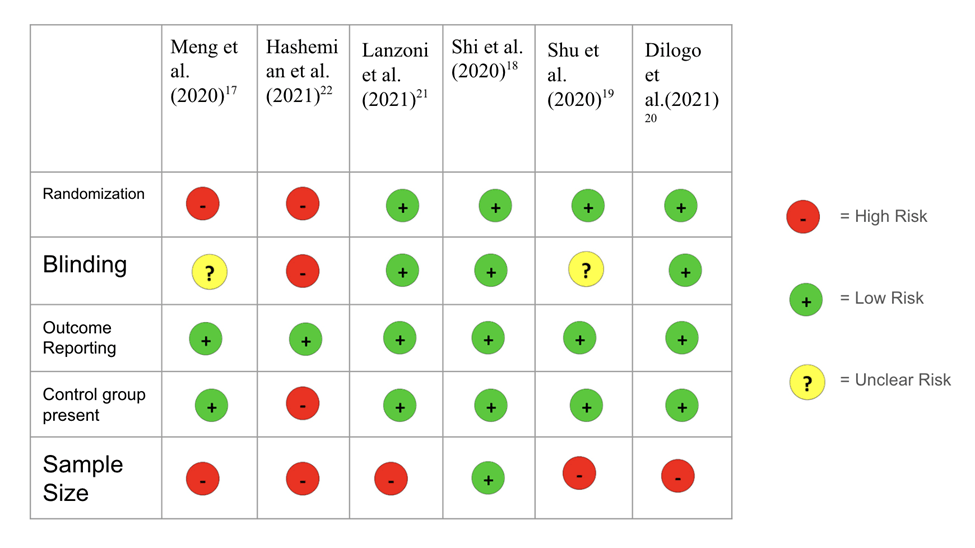
Risk of Bias (COVID-19 UC-MSC Trials)
Risk of bias was evaluated for all of the trials based on key methodological criteria such as randomization, blinding, outcome reporting, sample size, the variability of doses and if there was a control group present. Among the six studies reviewed, four were randomized studies (Lanzoni et al. (2021)22, Shi et al. (2020)23, Shu et al. (2020)24, Dilogo et al. (2021)25). The other two were non-randomized studies (Meng et al. (2020)20, Hashemian et al. (2021)21). Randomized studies generally indicate lower risk of bias due to its stronger study design. However, two out of the six are not randomized and the sample was taken by availability, introducing potential selection bias. Blinding decreases the risk of bias. Meng et al. (2020)20, Hashemian et al. (2021)21, Shu et al. (2020)24 had either no blinding or blinding was not mentioned. On the other hand, Lanzoni et al. (2021)22, Shi et al. (2020)23 and Dilogo et al. (2021)25 were all double-blind procedures, meaning neither the patient nor the assessing physician were aware of the treatment assignment. All trials reported the outcomes that were investigated. Sample sizes also need to be taken into consideration. Not only is there a variety of sample sizes across the studies, but all of the trials except Shi et al. (2020)23 have a relatively small sample size. This can increase risk of random error and may not be generalizable. Dosage also varied widely among the studies. While it doesn’t directly impact bias, it raises concerns about the consistency in the results. It can act as a source of confounding and complicates comparison between studies. In other words, as the studies use different levels of doses, it is hard to tell whether differences in patient outcomes are due to the treatment itself or the amount of the treatment used which limits the ability to attribute observed effects solely to UC-MSC therapy. Additionally, Hashemian et al. further lacked a control group, which also limits the ability to attribute outcomes solely to the MSC treatment. Table 3. Risk of Bias on UC-MSC Clinical Trials for COVID-19. The risk of bias ranged from low to high across studies. Randomized trials with control groups and appropriate sample sizes were assessed as having lower risk, while early-phase, non-randomized studies without controls were judged to have higher bias risk. A complete risk of bias table is provided to detail these assessments for each individual study.
In the Meng et al. (2020) phase I study, there were 9 patients in the experimental group and 9 patients in the control. There were found to be no serious adverse events related to the infusion of UC-MSCs. The only observation close to an adverse event is how two patients were noticed with transient facial flushing and fever immediately after the infusion, but resolved within 4 hours26. No abnormalities occurred in electrocardiography within the patients. A striking observation in this study is that during the follow-up, 4 patients (2 with moderate and 2 with severe) with high IL-6 levels showed a decline of IL-6 in 3 days27. Specific inflammatory cytokine levels were also observed: interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein 1 (MCP-1), interferon-inducible cytokine (IP-10), IL-22, interleukin 1 receptor type 1 (IL-1RA), IL-18, IL-8, and macrophage inflammatory protein 1-alpha (MIP-1). Within 14 days, all these levels were seen reduced. Out of all the patients, the ones with the highest levels of IL-6 showed the biggest drop of the cytokine and improved oxygenation index20.
Hashemian et al. (2021) phase I study also reported that UC-MSCs were safe for use in treating patients diagnosed with COVID-19 and ARDS21. This study included patients who had additional complications like diabetes, hypertension, chronic lymphocytic leukemia and cardiomyopathy21. This study, however, had no control group so the observed changes cannot be completely credited to the use of UC-MSCs. There were no serious adverse events 24 to 48 hours after MSC infusions. There were 11 patients in total. 7 out of the 11 experienced dyspnea relief and improved oxygenation within 48-96 hours of infusion. Out of these 7 patients, 5 were discharged within 2-7 days, 1 patient was discharged on Day 18 after renal and liver issues and 1 died of sudden cardiac arrest on Day 7. The other 4 patients with signs of multi-organ failure or sepsis died within 5-19 days. After the follow-up and long term recovery, all 6 patients were dyspnea free on Day 60 and CT imaging showed substantial lung recovery.
Lanzoni et al. (2021) study showed significant reductions in inflammatory markers only in the UC-MSC group. Additionally, the Day 28 survival rate in the UC-MSC group was significantly higher at 91%, compared to 42% in the control group. The treatment overall was found to be safe and had no infusion related adverse events. Plasma analysis also revealed that there were statistically significant reductions in inflammatory cytokines—including IFN-γ, IL-6, TNF-α, GM-CSF, and PDGF-BB—only in the UC-MSC treatment patients. This also suggests that the therapeutic difference was due to immunomodulation of the UC-MSCs rather than something else such as antiviral effects.
In this randomized and double blinded phase 2 trial, Shi et al. (2020) phase II study tested for safety and was conducted with 100 patients where 65 were given the UC-MSCs and 35 were given the placebo. All adverse events were found to be unrelated and no death occurred within the study. This was tested in patients with severe COVID-19 and lung damage. One of the primary outcomes—change in total lung lesion proportion from baseline to Day 28—showed a trend toward improvement even though it did not reach statistical significance (P = 0.080). There was, however, a significant reduction in solid component lung lesions (P = 0.043) observed in the UC-MSC group compared to placebo. Additionally, patients in the UC-MSC group showed numerically greater improvement in the 6-minute walk test (6-MWT). Overall, the study supports the potential of UC-MSCs as it has been seen to be safe and there are also early signs of efficacy through the effects that were observed. This shows that UC-MSCs may promote lung repair, even in the later stages of COVID-19. A larger phase 3 trial is needed to confirm these findings.
In the Shu et al. (2020) phase I/II study, C-reactive protein and IL-6 levels decreased greatly ever since day 3 of the cell infusion24. The Shu et al. (2020) phase II study aimed to measure effectiveness through clinical symptom improvement as well as indicators of the following: C-reactive protein, lymphocyte number, and interleukin 624. Both Shu et al. (2020) and Shi et al. (2020) of these studies found improvement in patients’ symptoms such as chest tightness, shortness of breath, and fatigue. Chest CT scans also indicated that inflammation in the lungs reduced significantly in the stem cell treatment group compared to the control group24. Also, none of the 12 patients who were treated with UC-MSCs required invasive ventilation. It was also observed that the mortality rate in the experimental group was 0% compared to the control group which was at 10.34%, though the difference was not statistically significant due to the small sample size. The treatment was also associated with reduced insulin needs in diabetic patients which also can imply broader systemic effects. The authors acknowledge limitations (small sample size, non-random allocation due to stem cell supply constraints, lack of mechanistic data, etc.) but nonetheless, UC-MSC research holds a promising future due to the early signs of improvement in the studies.
Many of these studies observed a decrease in cytokine levels. Similarly, IL-6 levels decreased in patients given the UC-MSCs treatment in the Dilogo et al. (2021) phase II study. Efficacy was measured by survival rates and length of ventilator usage for the patients25. Clinical improvement and serious adverse events were also considered to test efficacy. The results of this study showed 2.5 times higher survival rate in the experimental group than the control group, which was given normal saline25. Experimental stem cell groups received 2 intravenous infusions while the control group received 2 infusions of vehicle solution. The Dilogo et al. (2021) study also discovered increasing levels of the anti-inflammatory cytokine, IL-10, which is responsible for the activation of T-lymphocyte suppressor/regulator and decreasing levels of certain immune cells, like CD8‐CXCR3 and CD56‐CXCR3, signifying that cytokine storms are settling and the patient is improving25. Vascular Endothelial Growth Factor (VEGF) is a protein that stimulates the development of new blood vessels and this is essential for tissue repair in the lungs. In the control groups, VEGF went down. On the other hand, patients in the experimental group were discovered with better repair to damaged lungs because MSCs boosted VEGF circulation25. This quality of decreasing inflammatory cytokines was also seen in Lanzoni et al. (2021) phase I study, which tested UC-MSC infusions in COVID-19 with Acute Respiratory Distress Syndrome (ARDS)22. The findings of the study, such as decrease in inflammatory cytokines and fewer adverse events, support further investigation with a larger sample size. All studies’ experimental and control groups received standard of care.
Use of UC-MSCs in COPD
Chronic obstructive pulmonary disease has been an ongoing battle for many, having been around for over 200 years. Very early references to emphysema include Bonet’s description as “voluminous lungs” in 1679, Morgagni describing 19 cases of “turgid lungs” from air, and the illustration of emphysematous lungs by Baille in 178928. These show how COPD has been prevalent for 2 centuries as physicians take note of symptoms. Treatments have been investigated for many years, but there hasn’t been a successful outcome. While UC-MSCs have not been commonly used in COPD clinical trials, it is still an area to be explored for potential treatment. 2 completed studies from Clinicaltrials.gov were used for COPD.
Table 4. Completed UC-MSC Clinical Trials for COPD. This table shows different study designs that used UC-MSC therapy as a potential treatment option for COPD. Key features include study design, sample size, cell dosage, primary and secondary outcomes, follow-up duration, and statistical significance of reported findings. P-values below 0.05 were considered statistically significant.
| References | Study Design | Sample Size: Experimental Group | Sample Size: Control Group | Dosage | Primary and Secondary Outcomes | Follow-up Duration | Statistical Significance of results P-values less than 0.05 are considered statistically significant |
| Hoang et al. (2021)29 | Non-randomized, Phase I/II trial | 20 | 20 | 1 × 10⁶ cells/kg (patient body weight) | Primary: safety- adverse events Secondary: efficacy- Number of admissions General self-efficacy The number unscheduled outpatient visits due to symptoms of COPD Arterial blood gas analysis Respiratory functions Electrocardiogram Inflammatory response Cytokine analysis from patients’ plasma | Patients were followed up at 3 months, 6 months and 12 months after UC-MSC administration. There is also an additional 1 month safety follow-up conducted via telephone. | Actual p-values for the results are not yet reported |
| Bich et al. (2020)30 | Pilot (Phase 0) clinical trial | 20 | 0 | 1 × 10⁶ cells/kg (patient body weight) | Primary: safety- adverse events, vial signs and ECGs Secondary: Pulmonary function tests Exercise capacity Dyspnea Assessment Quality of life (COPD Assessment Test – CAT and St. George’s Respiratory Questionnaire (SGRQ) ) Systemic inflammation (CRP levels) COPD exacerbations | All patients were followed up for 6 months. Evaluations were conducted at 1 month, 3 months, and 6 months. | mMRC score at 3 month: 0.033 mMRC score at 1 month: 0.005 mMRC score at 6 month: 0.017 CAT score at 1 month: 0.002 CAT score at 3 month: 0.001 CAT score at 6 month: 0.003 Number of exacerbations at 6 months: <0.001 |
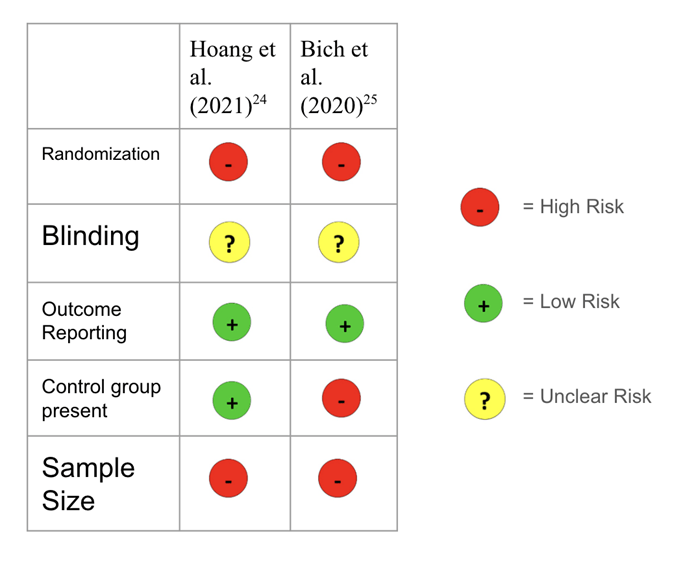
Risk of Bias (COPD UC-MSC Trials)
Risk of bias was evaluated for all of the trials based on key methodological criteria such as randomization, blinding, outcome reporting, sample size, the variability of doses and if there was a control group present. Among the two studies reviewed, both were non-randomized studies (Hoang et al. (2021)29, Bich et al. (2020)30), an indication of bias and a limitation in the COPD trials with UC-MSCs within this paper. Blinding was not mentioned for either of the trials. Both trials reported all outcomes that were meant to be examined.. Both of the studies have small sample sizes, increasing risk of random error and non-generalizability. Bich et al. (2020)30 did not have a control group, limiting the ability to attribute observed effects to the therapy. However, both studies in the COPD UC-MSC therapy studies have the same amount of dosage. This way, the amount of dosage cannot act as a confounding variable.
Table 5. Risk of Bias on UC-MSC Clinical Trials for COPD. The risk of bias remained relatively high for the COPD trials included in this paper. As there were only two studies taken Table 5. Risk of Bias on UC-MSC Clinical Trials for COPD. The risk of bias remained relatively high for the COPD trials included in this paper. As there were only two studies taken into consideration for COPD and UC-MSC trials, the risk of bias is higher. Both of the studies were also non-randomized and had small sample sizes because this therapy is still an emerging field. As there were only 2 studies conducted for UC-MSC therapy for COPD patients, the limited data restricts the knowledge available on the safety and efficacy of this treatment.
Hoang et al. (2021) is a study protocol that evaluates both safety and efficacy on this treatment. The primary outcome was measured through adverse events that were associated with the infusion of UC-MSCs or the treatment. The efficacy is measured through the number of admissions, arterial blood gas analysis and lung function. Lung function is examined through CT scans and chest X-rays. Previous trials of using bone marrow and umbilical cord derived mesenchymal stem cells have established safety but there are very inconsistent results on efficacy. This trial aims to build upon the pre-existing evidence of UC-MSCs’ abilities in regenerative medicine and to study the immunomodulatory effects of UC-MSCs on patient lymphocytes. Currently, there is no effective treatment for COPD, which is why investing research into UC-MSCs may be necessary to find a new therapeutic approach.
Bich et al. (2020) pilot study measured safety through the COPD assessment test, number of exacerbations and pulmonary function testing. UC-MSCs were prepared under stringent quality and safety standards. They were administered intravenously at a dose of 1.5 × 10⁶ cells/kg. The treatment was well tolerated over a 6-month period with no serious adverse effects reported. Although improvements in CRP levels and 6-minute walk test (6MWT) performance were observed, these did not reach statistical significance—likely due to the study’s small sample size. The study’s results concluded that UC-MSCs were safe in patients with moderate to severe COPD. The study also found that UC-MSCs were tolerated and safe30.
Both studies found significant improvement in some aspects of COPD, including mMRC, CAT and exacerbations. Modified Medical Research Council (mMRC) Dyspnea Scale is used to assess the level of functional disability caused by dyspnea. Dyspnea may also be referred to as “feeling short of breath.” CAT is the COPD Assessment Test which is a questionnaire for people with COPD about their health. The number of exacerbations also reduced after patients were treated with UC-MSC therapy. Hoang et al. suggested standard care for patients in both groups.
Discussion
All of these studies aimed to evaluate the safety of UC-MSC treatment and their potential efficacy. Their primary outcome that was used to evaluate safety was the number of adverse events. UC-MSCs treatment for COVID-19 patients was seen to be “safe and tolerable”20, after seeing that no adverse events occurred within the patients during the Meng et al. (2020) study. Hashemian et al. (2021) found no significant adverse effects that were associated with the UC-MSC treatment. Lanzoni et al (2021), on the other hand, was treating moderate to severe COVID-19 patients and adverse events were observed in the trial, although there was a lower amount of adverse events in the experimental group versus the control group. There were 2 serious adverse events in the experimental group, affecting 2 out of the 12 patients. On the other hand, there were 16 serious adverse events in the control group, affecting 8 out of the 12 patients. There were 2 deaths in the UC-MSC group and 7 deaths in the control group by day 28 after the second infusion. All subjects died of causes unrelated to their COVID-19 disease and the administration of the UC-MSC treatment. There were 2 studies that observed transient facial flushing and fever right after the infusion but it resolved spontaneously. This characteristic was observed in the studies Meng et al. (2020)20 and Hashemian et al. (2021)21. Hashemian et al. (2021) phase I study implemented UC-MSC treatment on patients with additional complications. The additional complications are correlated with signs of organ-failure and development of sepsis, suggesting that stem cell therapy may not be the ideal treatment for patients with these conditions. Additionally, there were signs of improvement as IL-6 levels decreased within patients, reinforcing the potential of UC-MSCs.
Table 6. Comparative Overview of UC-MSC Trial Characteristics and Outcomes. This table summarizes 6 clinical trials for UC-MSC use in COVID-19 with a focus on changes in IL-6 levels, survival benefits and adverse events. Across most of the studies, UC-MSC therapy was associated with reductions in IL-6 levels and minimal to no serious adverse events.
| References | IL-6 change | Survival benefit | Adverse Events |
| Meng et al. (2020)20 | Significant IL-6 reduction in treatment group by day 3 (p < 0.05) | No deaths reported | No serious adverse events; only transient facial flushing and fever in 2 patients after the infusion which resolved within 4 hours. |
| Hashemian et al. (2021)21 | IL-6 levels decreased in five (P=0.06) patients | 5 deaths out of 11 in total | No serious adverse events; two cases of shivering after infusion which resolved by supportive treatment in less than 1hr |
| Lanzoni et al. (2021)22 | Did not report | 2 deaths in the UC-MSC group and 7 in the control group. All deaths were unrelated to the infusion. | No serious adverse events related to the infusion were observed |
| Shi et al. (2020)23 | IL-6: 7.86 pg/mL (5.63–9.84) in UC-MSC group vs. 8.76 pg/mL (6.54–11.77) in placebo group | No deaths in this trial | The incidence of adverse events were similar in both of the groups but were found to be unrelated to the UC-MSC therapy |
| Shu et al. (2020)24 | IL-6 levels were significantly decreased from day 3 of stem cell infusion in the hUC-MSC group (no numerical value) | 3 deaths in total but P>0.05 so it is not statistically significant | No serious adverse events related to the infusion were observed |
| Dilogo et al.(2021)25 | Did not report | The survival rate was 35% (n=14). It was 2.5 times higher in the MSC group than the control group. | No serious adverse events related to the infusion were observed |
Through this table, it is observed how there were no serious adverse events and all deaths were unrelated to the treatment. There is a trend of decreasing IL-6 levels in the majority of the studies. Additionally, 5 patients in this trial (Hashemian et al.) showed a decrease in IL-6 levels with borderline statistical significance of P=0.06. In Shi et al. (2020) study, it is mentioned that serum IL-6 is one of the cytokines that played an important role in the development of COVID-19 disease. However, this trial found no statistical significance difference in the IL-6 levels between the UC-MSC group and the placebo group: IL-6 median values were 7.86 pg/ml (IQR: 5.63–9.84) in the UC-MSC group and 8.76 pg/ml (IQR: 6.54–11.77) in the placebo group. Even though Meng et al. (2020) phase I trial focused on safety, researchers noted bigger drops of cytokines levels and improved oxygenation index in patients with elevated levels of IL-6. One of the patients with a high baseline IL-6 level showed the most notable reduction in IL-6 and corresponding respiratory improvement. This links the decline of IL-6 levels and therapeutic benefit. This also shows us that this treatment might be more beneficial to patients with high levels of IL-6 rather than lower plasma IL-6 levels26. This might be because the inflammatory environment amplified the UC-MSCs’ immunomodulatory effects. Elevated levels of IL-6 are also associated with severe inflammation and cytokine storms, which are common in severe COVID-19 patients. These subsequent drops in IL-6 could suggest that UC-MSC treatment would be more beneficial for patients with severe COVID-19 or are experiencing heightened inflammatory responses. The specific cytokines mentioned also reduced in a matter of 14 days, indicating that the UC-MSCs therapy is on a successful path. Meng et al. (2020) phase I results indicate that multiple transfusions of the cells did not affect the patient’s severity of COVID-19 by aggravating the immune response.
During Shu et al. (2020) study, IL-6 and CRP levels, which are inflammatory cytokines, were seen to be reduced and lymphocyte count returned to normal levels in smaller amounts of time compared to the control group24. The reductions in IL-6 and CRP were reported as statistically significant starting from Day 3 post-infusion in the hUC-MSC group, although exact numerical values were not provided. This improvement in inflammatory markers indicates that UC-MSCs not only have the potential to alleviate inflammation but also restore immune function. Another discovery was made as well. Researchers noticed that people with COVID-19 who had diabetes used less exogenous insulin, suggesting that UC-MSCs therapy may be most efficient when used for COVID-19 patients with diabetes. This is especially because diabetes is a risk factor for death in COVID-19 patients24. Shu et al. (2020) highlighted this effect but they did not go into further detail about if this improvement of glycemic control was a survival benefit or not. They do acknowledge that their small sample size is limited so the ability to stratify by subgroups, including patients with comorbidities, was not possible and so that potential bias could not be excluded. Dilogo et al. (2021) and Lanzoni et al. (2021) both included critically ill COVID-19 patients with various comorbidities, including diabetes, hypertension, and obesity. But, neither of these two studies performed adjusted analyses to see if the survival benefits persisted after accounting for these underlying conditions. In Hashemian et al. (2021), a diabetic patient with cardiomyopathy survived and showed clinical improvement after UC-MSC infusion, including a significant drop in liver enzymes. But as this was one singular case and obviously conclusions about broader survival effects in comorbid subgroups cannot be drawn.This presents a key limitation but it also means that there is a new avenue for research with UC-MSCs. The Shu et al. (202) study found that UC-MSC therapy is most efficacious in patients with severe COVID-19. In another study, combined treatment (mesenchymal stromal cells and standard regimen) was most efficacious as it improved survival rates for critically ill patients25. The Lanzoni et al. (2021) study tested COVID-19 patients with ARDS while the Dilogo et al. (2021) study tested critically-ill COVID-19 patients24,22. Both studies had similar results to Shu et al. (2020) study, supporting that UC-MSC treatment was more beneficial towards COVID-19 patients who were in a critical stage and/or had other complications (like diabetes or ARDS) which worsened their condition. Another case series study, Hashemian et al. (2021), tested for the safety of UC-MSC treatment in patients with COVID-19 and ARDS. The study results presented that this treatment was safe for use. These studies also found a decrease in IL-6 levels after application of UC-MSCs. The findings from the Dilogo et al. (2021) study regarding IL-10 levels and VEGF circulation highlight improvements on the immune response and lung tissue repair in patients. The increased anti-inflammatory IL-10 suggests that the treatment can help stabilize the immune system by reducing hyperactivity and inhibiting cytokine storms. Patients who received the treatment have better VEGF circulation, which is needed for angiogenesis and regenerating damaged lung tissue. These results underscore how UC-MSCs can help with immune system modulation and lung tissue regeneration.
Similarly, UC-MSC treatment studies for COPD patients had safe results. Hoang et al. (2021) and Bich et al. (2020) both supported UC-MSC therapy being safe. Bich et al. (2020) provides data from mMRC and CAT tests along with number of exacerbations, supporting the safety and therapeutic potential of UC-MSCs in treating COPD. Hoang et al. (2021) provides projective results that encourage further investigation of UC-MSCs’ use on COPD patients. The improvements in assessments like the mMRC and CAT show the treatments’ ability to improve quality of life by reducing disability dyspnea. The reduction in frequency of exacerbations following UC-MSC treatment can potentially mean reducing the need for hospitalization and emergency interventions. These results indicate that UC-MSC treatment is safe but this still needs to be further researched with a larger sample size and more trials. After safety is guaranteed, UC-MSCs can be researched further for efficacy. As there were only 2 of these COPD studies available that investigated safety, more safety trials are the next step. Overall, there was no harm seen in using UC-MSCs in patients for both respiratory diseases.
Across all the studies, there is one setback: inconsistent dosing. Dosing plays a critical role in the therapeutic outcomes of UC-MSCs for any disease, including the ones discussed in this paper—COPD and COVID-19. These studies have used a variety of different dosing amounts including fixed cell numbers and calculated dosage based on weight. There is also a mix of studies that transplanted UC-MSCs with a single infusion versus multiple infusions. For instance, Meng et al. (2020)26 administered three cycles of 3 × 10⁷ cells per infusion and the study observed clinical improvement without any adverse events. This suggests that multiple doses were tolerated well and is potentially beneficial. On the other hand, Hashemian et al. (2021)21 used a higher cumulative dose of 600 × 10⁶ cells over three infusions, specifically in critically ill ARDS patients and reported rapid clinical responses in 48-96 hours after the infusion even though the study did not have a control group. Lanzoni et al. (2021) used a moderate fixed dose and found significantly improved survival and reduced inflammation. Similar to the repeated dosing in Lanzoni et al. (2021), Shi et al. (2020) used 3 infusions of 4 × 10⁷ cells each and reported significant improvements in lung imaging and inflammatory markers. This further confirms the potential advantage of repeated medium-dose administration. Shu et al. (2020) and Dilogo et al. (2021) employed weight-based dosing. They found that single infusions are also safe and efficacious, specifically when they are administered in an earlier stage of the disease. Overall, repeated moderate doses seem to yield the strongest combination of safety and clinical benefits (Shi et al. & Lanzoni et al.). High doses also seem to have immediate effects on the patients but further investigation is needed because those studies have not been compared to a control group and only followed up with the patient for 60 days so long-term efficacy is still inconclusive (Hashemian et al.). Lower weight based doses were also seen to be very safe and there were some significant improvements, especially if it was given in earlier stages of COVID-19, but there is a possibility that it will not be effective in patients with severe COVID-19. However, more research on the best dosing quantity is still needed.
Standard care was also administered to all of the patients across the studies for COVID-19 and it typically included antivirals, corticosteroids (such as dexamethasone), antibiotics, anticoagulants, and oxygen support. For COVID-19 trials, this background therapy plus the institutional or national COVID-19 treatment guidelines was, either implicitly or explicitly, provided in both the UC-MSC and control groups. For example, Meng et al., Hashemian et al., and Shi et al. clearly stated that all patients received recommended standard COVID-19 regiments. Shu et al. had a detailed standard of care involving supplemental oxygen, antivirals (abidor/oseltamivir), antibiotics and glucocorticoids. Lanzoni et al. noted how their standard of care evolved over time to better fit the patients’ needs but it was evenly distributed through stratified randomization. Dilogo et al. administered azithromycin and oseltamivir along with the requirements of the guidelines. However, this co-administration of UC-MSCs alongside with the standard of care treatment makes it hard to solely attribute the effects of the treatment to UC-MSCs. Shi et al. also notes how the use of UC-MSC as an adjunctive therapy to standard care is also an effective option to be considered. But even in randomized trials like Shi et al. and Lanzoni et al., observed benefits like shorter recovery times or proinflammatory markers decreasing may be partially due to the addition of standard care treatment. So overall, while the clinical outcomes seem promising, giving the credit to UC-MSCs may not be valid till there are further trials that can study the isolated effect of UC-MSCs. But for now, combined therapy has pointed in a positive direction. For the COPD trials, Hoang et al. suggested the use of standard care for both groups based on the GOLD 2019 guidelines and national protocols as there is no current set treatment for COPD. However, Bich et al. did not explicitly state any information about any background treatment, making the origins of the effects of the study unclear.
While early UC-MSC trials point towards success, whether this therapy is better than other treatments is another discussion. As mentioned earlier in the review, corticosteroids and antiviral agents are also other forms of treatment. Corticosteroids, such as dexamethasone, are commonly used to reduce inflammation. They have also shown some mortality benefits in critically ill COVID-19 patients31. However, corticosteroids can have broad immunosuppressive effects and increase the risk of secondary infections. In contrast, UC-MSCs exert targeted immunomodulatory effects by secreting anti-inflammatory cytokines and promoting immune balance without broadly suppressing the immune response. Additionally, antiviral therapies have been seen to have inconsistent efficacy and this can typically be seen in severe COVID-19 cases32.
Unlike corticosteroids or antivirals, UC-MSCs have the distinct ability of tissue regeneration, which becomes very relevant when it comes to lung repair in both COVID-19-induced ARDS and COPD. Preliminary clinical trials for UC-MSCs suggest safety. And while UC-MSC therapy remains in early-phase trials, its potential for immune regulation and tissue repair is essential to consider.
Beyond all the clinical aspects of this treatment, cost effectiveness is also a factor that needs to be considered. UC-MSC therapy also has a very promising future for application in chronic inflammatory diseases like COPD and COVID-19. However, it is still costlier than other traditional treatments like corticosteroids. Corticosteroids are inexpensive and easily available, thus a treatment people end up with. However, they are not very effective and UC-MSCs need more studies to be done so that its complete potential is realized. Thus in contrast to corticosteroids, UC-MSCs present a novel therapeutic approach with immunomodulatory activity, and while more expensive than corticosteroids, they are comparatively cost-effective in relation to other advanced therapy medicinal products (ATMPs). For example, chimeric antigen receptor T-cell therapies such as Kymriah and Yescarta cost around €350,000 per patient. Luxturna costs around €800,000. And Zolgensma remains the most expensive therapy, with a cost of €1.9 million per patient. In comparison, MSC-based therapies are significantly more affordable: Alofisel costs €54,000 in France and Temcell was approved in Japan at a maximum price of $104,00011. Manufacturing estimates for allogeneic MSC-based therapies range between €15,000 and €30,000 per patient11, which is considerably lower than other ATMPs. And beyond all of this, UC-MSCs can be mass-produced from a readily available source which is also ethically noncontroversial and most of all is unlimited: umbilical cord tissue. This allows for the creation of scalable “off-the-shelf” products, further reducing costs11. As it can be seen through the data there are multiple factors that collectively support the long-term cost effectiveness of the UC-MSC therapy, especially as its research continues to expand.
Conclusion
In summary, UC-MSC therapy is concluded to be safe. While efficacy is still being researched, the treatment is safe for further use. UC-MSC research in the COVID-19 disease has been more successful as the immunomodulatory effects were clearly presented in the patients that received this treatment. An increase in anti-inflammatory cytokines also aided with the healing of damaged lungs. This therapy was more effective in COVID-19 patients with diabetes or ARDS. Meanwhile, the next steps for UC-MSC treatment in COPD patients is to be further researched for efficacy. While these studies show successful results, further research is needed to fully evaluate the long-term efficacy of UC-MSC treatment in both diseases.
References
- Umakanthan, S., Sahu, P., Ranade, A. V., Bukelo, M. M., Rao, J. S., Abrahao-Machado, L. F., Dahal, S., Kumar, H., & KV, D. (2020, June 20). Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). NCBI. Retrieved August 7, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10016932/ [↩]
- Zanza, C., Romenskaya, T., Manetti, A. C., Franceschi, F., Russa, R. L., Bertozzi, G., Maiese, A., Savioli, G., Volonnino, G., & Longhitano, Y. (2022, January 18). Medicina | Free Full-Text | Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. MDPI. Retrieved August 7, 2024, from https://www.mdpi.com/1648-9144/58/2/144 [↩] [↩] [↩] [↩] [↩] [↩]
- Cao, B., Wang, Y., Wen, D., Liu, W., Wang, J., Fan, G., Ruan, L., Song, B., Cai, Y., Wei, M., Li, X., Xia, J., Chen, N., Xiang, J., Yu, T., Bai, T., Xie, X., Zhang, L., Li, C., … Wang, C. (2020). A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. New England Journal of Medicine, 382(19), 1787–1799 from https://www.nejm.org/doi/full/10.1056/NEJMoa2001282 [↩]
- Faist, A., Janowski, J., Kumar, S., Hinse, S., Çalışkan, D. M., Lange, J., Ludwig, S., & Brunotte, L. (2022, July 14). Virus Infection and Systemic Inflammation: Lessons Learnt from COVID-19 and Beyond. NCBI. Retrieved August 16, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9316821/ [↩]
- Huerta, F. S., Martinez-Guerra, B. A., Roman-Montes, C. M., Tamez-Torres, K. M., Rajme-Lopez, S., Ortíz-Conchi, N., López-García, N. I., Villalobos-Zapata, G. Y., Rangel-Cordero, A., Santiago-Cruz, J., Xancal-Salvador, L. F., Méndez-Ramos, S., Ochoa-Hein, E., Galindo-Fraga, A., Ponce-de-Leon, A., Gonzalez-Lara, M. F., & Sifuentes-Osornio, J. (2023). Risk Factors Associated with the Development of Hospital-Acquired Infections in Hospitalized Patients with Severe COVID-19 from https://pmc.ncbi.nlm.nih.gov/articles/PMC10376785/ [↩]
- Boers, E., Barret, M., Su, J. G., Benjafield, A. V., Sinha, S., Kaye, L., Zar, H. J., Vuong, V., Tellez, D., Gondalia, R., Rice, M. B., Nunez, C. M., Wedzicha, J. A., & Malhotra, A. (2023, December 7). Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. NCBI. Retrieved August 7, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10704283/ [↩] [↩]
- Barbu, C., Iordache, M., & Man, M. G. (2011). Inflammation in COPD: pathogenesis, local and systemic effects. RJME: Romanian Journal of Morphology & Embryology. Retrieved August 11, 2024, from https://www.rjme.ro/RJME/resources/files/520111021027.pdf [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Higham, A., Mathioudakis, A., Vestbo, J., & Singh, D. (2020, November 4). COVID-19 and COPD: a narrative review of the basic science and clinical outcomes. NCBI. Retrieved August 16, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7651840/ [↩] [↩]
- Coelho, A., Alvites, R. D., Branquinho, M. V., Guerreiro, S. G., & Maurício, A. C. (2020, November 19). Mesenchymal Stem Cells (MSCs) as a Potential Therapeutic Strategy in COVID-19 Patients: Literature Research. NCBI. Retrieved August 20, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7710935/ [↩] [↩] [↩]
- Hossein-khannazer, N., Shokoohian, B., Shpichka, A., Aghdaei, H. A., Timashev, P., & Vosough, M. (2020, June 3). Novel therapeutic approaches for treatment of COVID-19. NCBI. Retrieved August 20, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7268974/ [↩] [↩] [↩]
- Mebarki, M., Abadie, C., Larghero, J., & Cras, A. (2021, February 26). Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products – Stem Cell Research & Therapy. Stem Cell Research & Therapy. Retrieved August 11, 2024, from https://stemcellres.biomedcentral.com/articles/10.1186/s13287-021-02222-y [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Jarczak, D., & Nierhaus, A. (2022, October 3). Cytokine Storm—Definition, Causes, and Implications – PMC. NCBI. Retrieved August 11, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9570384/ [↩]
- Kany, S., Vollrath, J. T., & Relja, B. (2019, November 28). Cytokines in Inflammatory Disease – PMC. NCBI. Retrieved August 11, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6929211/ [↩]
- Makaremi, S., Kianfar, H., Asgarzadeh, A., & Mohammadnia, A. (2022, June 25). The role of IL-1 family of cytokines and receptors in pathogenesis of COVID-19. ResearchGate. https://link.springer.com/article/10.1007/s00011-022-01596-w [↩]
- Wang, Y., Xu, J., Meng, Y., Adcock, I. M., & Yao, X. (2018, October 12). Role of inflammatory cells in airway remodeling in COPD. NCBI. Retrieved August 11, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6190811/ [↩] [↩]
- Meng, M., Zhang, W.-W., Chen, S.-F., Wang, D.-R., & Zhou, C.-H. (2024, February 26). Therapeutic utility of human umbilical cord-derived mesenchymal stem cells-based approaches in pulmonary diseases: Recent advancements and prospects. NCBI. Retrieved August 20, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10915951/ [↩] [↩]
- Hoang, D. M., Pham, P. T., Bach, T. Q., Ngo, A. T. L., Nguyen, Q. T., Phan, T. T. K., Nguyen, G. H., Le, P. T. T., Hoang, V. T., Forsyth, N. R., Heke, M., & Nguyen, L. T. (2022, August 6). Stem cell-based therapy for human diseases. NCBI. Retrieved August 11, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9357075/ [↩] [↩]
- Hori, A., Takahashi, A., Miharu, Y., Yamaguchi, S., Sugita, M., Mukai, T., Nagamura, F., & Nagamura-Inoue, T. (2024). Superior migration ability of umbilical cord-derived mesenchymal stromal cells (MSCs) toward activated lymphocytes in comparison with those of bone marrow and adipose-derived MSCs. Frontiers in Cell and Developmental Biology, 12. [↩]
- Strecanska, M., Sekelova, T., Csobonyeiova, M., Danisovic, L., & Cehakova, M. (2024). Therapeutic applications of mesenchymal/medicinal stem/signaling cells preconditioned with external factors: Are there more efficient approaches to utilize their regenerative potential? Life Sciences, 346, 122647. [↩]
- Meng, F., Xu, R., Wang, S., Xu, Z., Zhang, C., Li, Y., Yang, T., Shi, L., Fu, J., Jiang, T., Huang, L., Zhao, P., Yuan, X., Fan, X., Zhang, J.-Y., Song, J., Zhang, D., Jiao, Y., Liu, L., … Wang, F.-S. (2020, August 27). Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. NCBI. Retrieved August 21, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7450163/ [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Hashemian, S.-M. R., Aliannejad, R., Zarrabi, M., Soleimani, M., Vosough, M., Hosseini, S.-E., Keshel, S. H., Naderpour, Z., Hajizadeh-Saffar, E., Shajareh, E., Jamaati, H., Soufi-Zomorrod, M., Khavandgar, N., Alemi, H., Karimi, A., Pak, N., Rouzbahani, N. H., Nouri, M., Sorouri, M., … Baharvand, H. (2021, January 29). Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. NCBI. Retrieved August 21, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7844804/ [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Lanzoni, G., Linetsky, E., Correa, D., Cayetano, S. M., Alvarez, R. A., Kouroupis, D., Gil, A. A., Poggioli, R., Ruiz, P., Marttos, A. C., Hirani, K., Bell, C. A., Kusack, H., Rafkin, L., Baidal, D., Pastewski, A., Gawri, K., Leñero, C., Mantero, A. M. A., … Ricordi, C. (2021, January 5). Umbilical cord mesenchymal stem cells for COVID‐19 acute respiratory distress syndrome: A double‐blind, phase 1/2a, randomized controlled trial. NCBI. Retrieved August 21, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8046040/ [↩] [↩] [↩] [↩] [↩] [↩]
- Shi, L., Huang, H., Lu, X., Yan, X., Jiang, X., Xu, R., Wang, S., Zhang, C., Yuan, X., Xu, Z., Huang, L., Fu, J.-L., Li, Y., Zhang, Y., Zhang, B., Shi, M., Meng, F., Song, Y., Yu, Y., … Wang, F.-S. (2021, February 10). Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. NCBI. Retrieved August 21, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7873662/ [↩] [↩] [↩] [↩] [↩]
- Shu, L., Niu, C., Li, R., Huang, T., Wang, Y., Huang, M., Ji, N., Zheng, Y., Chen, X., Shi, L., Wu, M., Deng, K., Wei, J., Wang, X., Cao, Y., Yan, J., & Feng, G. (2020, August 18). Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. NCBI. Retrieved August 21, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7432540/ [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Dilogo, I. H., Aditianingsih, D., Sugiarto, A., Burhan, E., Damayanti, T., Sitompul, P. A. S., Mariana, N., Antarianto, R. D., Liem, I. K., Kispa, T., Mujadid, F., Novialdi, N., Luviah, E., Kurniawati, T., Lubis, A. M.T., & Rahmatika, D. (2021, June 8). Umbilical cord mesenchymal stromal cells as critical COVID‐19 adjuvant therapy: A randomized controlled trial. NCBI. Retrieved August 21, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8242692/ [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Meng, F., Xu, R., Wang, S., Xu, Z., Zhang, C., Li, Y., Yang, T., Shi, L., Fu, J., Jiang, T., Huang, L., Zhao, P., Yuan, X., Fan, X., Zhang, J.-Y., Song, J., Zhang, D., Jiao, Y., Liu, L., … Wang, F.-S. (2020, August 27). Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. NCBI. Retrieved August 21, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7450163/ [↩] [↩] [↩]
- Meng, F., Xu, R., Wang, S., Xu, Z., Zhang, C., Li, Y., Yang, T., Shi, L., Fu, J., Jiang, T., Huang, L., Zhao, P., Yuan, X., Fan, X., Zhang, J.-Y., Song, J., Zhang, D., Jiao, Y., Liu, L., … Wang, F.-S. (2020, August 27). Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. NCBI. Retrieved August 21, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7450163/ [↩]
- Petty T. L. (2006). The history of COPD. International journal of chronic obstructive pulmonary disease, 1(1), 3–14. [↩]
- Hoang, D. M., Nguyen, K. T., Nguyen, A. H., Nguyen, B. N., & Nguyen, L. T. (2021). Allogeneic human umbilical cord-derived mesenchymal stem/stromal cells for chronic obstructive pulmonary disease (COPD): study protocol for a matched case-control, phase I/II trial. BMJ open, 11(5), e045788. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8126295/ [↩] [↩]
- Bich, P. L. T., Thi, H. N., Chau, H. D. N., Van, T. P., Do, Q., Khac, H. D., Van, D. L., Huy, L. N., Cong, K. M., Ba, T. T., Minh, T. D., Bich, N. V., Chau, N. T., & Pham, P. V. (2020, February 13). Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. NCBI. Retrieved September 29, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7020576/ [↩] [↩] [↩] [↩]
- Meybodi, S. M., Rabori, V. S., Salkhorde, D., Jafari, N., Zeinaly, M., Mojodi, E., Kesharwani, P., Saberiyan, M., & Sahebkar, A. (2024, October 29). Dexamethasone in COVID-19 treatment: Analyzing monotherapy and combination therapy approaches. PubMed. [↩]
- Amani, B. (2024, June 7). Azvudine versus Paxlovid in COVID-19: A systematic review and meta-analysis. PubMed. [↩]