Abstract
Autism spectrum disorder is a neurodevelopmental disorder characterized by impairment in motor, social, and sensory functions. Considering that atypical microbiota and gastrointestinal disturbances compromise general health and exacerbate stereotypic behaviors, the nutrition problems an autistic child faces will be far more complicated than those of a typically developing child. These circumstances call for specific nutritional interventions to manipulate the composition of the diet to alleviate autistic manifestations. This article reviews the possible application of nanoparticle delivery systems in treating iron deficiency in autistic children. Literature regarding the application of nanoparticles in nutritional interventions for children with ASD was referred to in databases of Google Scholar and PubMed. Full texts that were published within the last 15 years were reviewed critically for their contents. 23 abstracts were reviewed, 22 full papers were reviewed, and 12 papers were included in the study; 6 in vivo, 6 in ID in ASD. It is further evident that conventional oral iron supplementation is neither a safe nor an effective treatment option for this population, given its potential to exacerbate any preexisting gastrointestinal problems. Nanoparticles have been demonstrated as a potential novel approach to managing gastrointestinal disorders associated with autism while rectifying iron deficiency and minimizing gastrointestinal side effects. This article explores how nanoparticles present a great potential as an alternative treatment for iron deficiency in children with ASD, which may decrease stereotyped behavior and improve general health in these children.
Keywords: Autism spectrum disorder, nanoparticles, iron deficiency, delivery system, nutrition deficiency
Introduction
Autism, or autism spectrum disorder (ASD), is a complex developmental condition involving persistent challenges with social communication, restricted interests, and repetitive behavior1. The prevalence of ASD is 2.5% among US children2, and approximately 1/100 children are diagnosed with ASD around the world3. This growing prevalence underscores the urgent need for innovative treatment strategies that address not only the core symptoms of autism but also its associated comorbidities, such as iron deficiency anemia (IDA). Iron deficiency is the most prevalent single-nutrient deficiency worldwide and results in anemia, decreased immune function, retarded growth and impaired thermoregulation4, and ultimately, irreversible neurocognitive and behavioral deficits5,6. Specifically, IDA may lead to impaired cognitive function, slowed growth, impaired concentration, and mood changes in autistic children7.
IDA is prevalent among children with autism, and they are at a higher risk compared to neurotypical peers7,8,9. A study in Turkey found that in children with autism, 24.1% of them had iron deficiency, and 15.5% had anemia10. A study led by Latif et al. in the UK also showed a high prevalence of 52% with low serum ferritin among ASD children7 and Dosman et al. in Canada reported a higher prevalence of low ferritin11, confirming that iron deficiency and anemia are common in children with autistic disorder. The contributing factors that may predispose children with ASD to iron deficiency include the relationship between dietary iron intake and ferritin. In a human observational study, there was a statistically significant association between low ferritin and food preferences (ie, color, texture), indicating that dietary behaviors might influence nutritional status. Additionally, these dietary restrictions may contribute to GI issues like constipation or diarrhea12,13. In addition, some children with ASD may have deficiencies in digestive enzymes, which can impact the breakdown of food components. This inadequate enzyme activity can lead to malabsorption and GI disturbances14.
Currently, ASD treatments are largely focused on physical or psychiatric aspects, leaving patients burdened by the gastrointestinal disorders that impact patients with ASD. The dysfunction of absorption and special gastrointestinal disorders that children with autism suffer from result in impaired quality of life, and addressing these issues can improve the overall well-being of children with ASD, leading to better behavior, cognitive function, and educational abilities13. Current treatments for IDA typically involve oral iron supplements; however, these can exacerbate gastrointestinal (GI) disturbances15, which are particularly problematic for autistic individuals who may already experience heightened sensitivities.
The advent of nanoparticle technology offers a promising alternative for delivering iron supplements more effectively while minimizing side effects. Nanoparticles are tiny materials with a size range from 1 to 100 nanometers (nm). This nanoscale size gives them special physical and chemical properties due to the high surface area16, allowing them to be widely utilized in food fortifications17 and drug delivery systems18,19. Nanoparticles make iron more bioavailable and reduce GI discomfort via targeted delivery throughout the body. Nanosized iron preparation can resolve the issues of GI disturbances and malabsorption. The surface area of iron compounds is increased by reducing their particle size, which improves their solubility in gastric juice and boosts their absorption20, improving the bioavailability of active ingredients and introducing controlled and targeted release21.
Studies have demonstrated that iron nanoparticles are efficient compared to iron salts in treating anemia and are relatively non-toxic compared to the latter22. However, despite this potential, there is very scarce literature on the use of nanoparticles for the treatment of IDA in autistic populations, and there are no reviews about their use in this respect within the context of autism.
To address this gap, this paper uses primary and secondary research to explore the possibility of nanoparticles for treating autistic children with IDA in the form of a literature review. This analysis will underscore the need for research focusing on the unique physiological characteristics of autistic children in nutritional supplements.
Methods
We conducted three literature searches to address the following aims: (1) identify primary human clinical studies that discuss the prevalence, risk, and current interventions for iron deficiency anemia in ASD, (2) in vitro studies that evaluate the use of nanoparticles in populations other than those with ASD, and (3) human experimental studies that evaluate the use of nanoparticles in ASD. Each investigation was conducted under unique requirements for their own purpose, and the whole reviewing process is shown in the chart.
Human Experimental Studies (IDA in ASD)
We conducted a literature review of primary sources (human clinical studies) in Google Scholar and PubMed databases from 2003 to 2024, using the search terms “iron deficiency” and “autism spectrum disorder”.
Titles and abstracts were reviewed to determine relevance. Abstracts were excluded if they did not include primary data about iron deficiency anemia in ASD. Six studies were selected for full review. Of the six studies, one study was excluded for not providing a quantitative evaluation of IDA outcomes. Five papers studying autistic symptoms related to iron supplement treatment were selected for inclusion in this review. The review of primary sources ensures that the discussion focuses on iron deficiency anemia, exploring the potential for nanoparticles in the delivery system.
In Vitro Studies (Nanoparticles in ASD)
We conducted a second literature search for in vivo experimental studies using nanoparticles to deliver iron supplements in populations not specific to ASD. The inclusion/exclusion criteria for this search were as follows:
Inclusion:
- The study discusses the use of nanoparticles and iron deficiency status
- The study method includes case study, case-control, randomized controlled trials, and systematic reviews.
- Provide specific statistics for the outcomes of the experiments(hemoglobin, hematocrit, iron, ferritin, MCV, and RDW).
Exclusion:
- The subjects of the study do not have iron deficiency anemia
- The study was not published in English
- The piece is a book chapter.
Each paper’s abstract was first reviewed to ensure the study’s relevance and confirm access. Then they are assessed to see whether they satisfied the above inclusion and exclusion criteria. We reviewed 16 full papers, and 6 were included in the final analysis; the rest were excluded for not satisfying the inclusion requirements and not providing accessible data.
Human Clinical Studies (Nanoparticles in ASD)
We conducted a third literature review using the keywords “GI disorders”, and “application of nanoparticles”. Abstracts were reviewed to ensure relevance to ASD or IDA, reference to primary studies, and to draw quantitative conclusions about the relationship between ASD and IDA (using the outcome metrics above).
Studies chosen for inclusion were evaluated for the following components:
- The content is highly relevant to power the analysis
- A study design appropriate for the proposed research question (case-control, randomized controlled trial)
Only studies that met this quality assessment were included for full review.
Data Analysis and Presentation
Before data analysis, all included studies were screened to identify a clear risk of bias, such as lack of randomization, large lost-to-follow-up rates, and appropriate treatment of confounders, etc. These biases were noted and discussed in the discussion section.
The data of the included studies were recorded using a spreadsheet recording the citation, keywords, the methodology, and the primary outcomes, separated by study type. The main outcomes assessed include the association between ASD and Iron deficiency and changes in the concentration of iron markers like hemoglobin, hematocrit, iron, ferritin, MCV, and RDW. Outcomes were tabulated and visualized using Microsoft products.
Nanoparticles Overview
Various nanoparticles have been developed to treat IDA, including iron (Fe) oxide nanoparticles, iron solids, lipid nanoparticles, iron oxide magnetic nanoparticles-loaded liposomes, and zinc oxide nanoparticles. Nanoparticles are synthesized using different techniques, such as flame spray pyrolysis, co-precipitation, and hot homogenization/ultrasonication. It is very challenging to form iron into a micronutrient for fortification in food because of its high reaction rate with the different active substances within the food matrix. Compared with conventional iron fortificants, nanoparticles are better tolerated in the gut lumen and have superior bioavailability than conventional iron fortificants23. The reduction in particle size of iron compounds saves the esophagus and gastrointestinal tract from harsh effects, increases the surface area of iron salts, increases the solubility of the gastric juices, and increases absorption24. The use of nanosized iron compounds produces less organoleptic change in food vehicles than that caused by water-soluble iron complexes. The absorption of Fe in the gastrointestinal tract is good in the absence of comorbidities23. However, several safety concerns about nanoparticles exist, especially regarding oral exposure or their use as fortificants, which require more in vivo studies to determine nanoparticles’ safety when applied.
| Reference | Title | Sample / Population | Research Design (experimental, literature review, scoping review, etc.) | Main Outcome |
| Scout McWilliams et al. | Iron deficiency and common neurodevelopmental disorders—A scoping review | A literature search using the search terms “iron deficiency an(a)emia” AND “ADHD” OR “autism” OR “FASD”, on February 3rd, 2017. | Scoping review | Intellectual disability |
| An association of ID and ASD / ASD severity | ||||
| Selective eating habits and gastrointestinal comorbidities | ||||
| Dosman et al. | Children with Autism: effect of iron supplementation on sleep and ferritin. | 33 participants who completed the study (27 males, 6 females), mean age was 6 years, 6 months (2 years, 8 months to 10 years, 9 months) | Experimental Study | Exhibiting gastrointestinal symptoms and malabsorption syndrome |
| A relation between iron deficiency and sleep disorders in the ASD children | ||||
| Latif et al. | Iron Deficiency in Autism and Asperger Syndrome. | Measurements of 96 children (52 with autism and 44 with Asperger syndrome) | Experimental Study | Impaired cognitive function, slowing growth, impaired concentration, and mood changes in autistic children may be associated with iron deficiency or anemia. |
| Many of the ASD children had low serum ferritin, which may be a factor in the development of ASD disorders in this population grouping. | ||||
| Abbott R. | The effects of iron supplementation on cognitive function in infants and children. | Children and adolescents in schools in Austria | Experimental Study | Prevent them from receiving sufficient stimulus and developing new skills |
| ID/IDA may increase the severity of autistic symptoms in children with ASD. | ||||
| Grantham-McGregor S et al. | Poorer behavioral and developmental outcomes more than 10 years after treatment for iron deficiency in infancy. | 191 participants, 87% were reevaluated at 11 to 14 years old | Experimental Study | Negative effects on brain development |
| Social interaction problems and learning disabilities |
Results
Improving IDA could mitigate symptoms in children with ASD
Several studies have verified that children with autism have an increased risk of iron deficiency. The prevalence of iron deficiency among children with ASD is 7.5%, and hemoglobin levels in children with ASD are lower than those among normal children8. Furthermore, the adverse effects of IDA on brain development have been widely accepted, and behavioral problems in children with IDA have been expected more often24,5. In one study that attempted to assess the association of low serum ferritin with sleep disorders in children with autism (n=33), 77% of parents of autistic children who have IDA reported that their children have suffered from sleep disorders and gastrointestinal symptoms, suggesting malabsorption as a possible cause of iron deficiency in autism. When the children with sleep disorders and autism were treated with iron supplementation, improvement in sleeping behavior was reported25. IDA also leads to the cognitive impairment, growth retardation, concentration problems, and mood changes, which can further deteriorate their already damaged means of communication and behavior during development26,7.
Common iron supplementation typically requires a dose of 60-120 mg of elemental iron per day to be effective27,28. Currently, many common iron oral supplements, such as ferrous iron salts, polysaccharide-iron complex, and heme iron, are widely used. However, much research has indicated many side effects, including constipation, nausea, and vomiting29.
Additionally, there’s an obvious association between patients with high rates of GI issues with taking oral iron formulations, including ferrous fumarate, ferrous gluconate, and ferrous sulfate. This association might be due to the production of free radicals that can lead to inflammation and the changes to the gut microbiota composition or metabolism15,30.
Improved digestion and absorption of iron via nanoparticles
The commercial iron supplements often prove unsuitable for children with ASD due to their inherent gastrointestinal (GI) issues and malabsorption tendencies30,13. Studies show that children with ASD usually exhibit poorer malabsorption and experience more GI disorders. GI dysfunctions, including constipation, diarrhea, and abdominal pain. The prevalence of GI symptoms in children with ASD has been reported to range from 9 to 70% and higher31, and these symptoms can be exacerbated by traditional iron supplements. Unusual sleep, oppositional behavior, and rigid-compulsive behaviors have all been found to be significantly associated with GI problems among children with ASD32. This special situation, with the potential for exacerbating GI distress, raises concerns and renders conventional treatments less effective. Therefore, there is an urgent need for alternative treatments for iron deficiency in this group. Nanoparticles are a promising alternative method due to enhanced absorption and reduced side effects in the GI tract. Besides improving absorption in the intestinal tract due to their high surface-to-volume ratio, they may reduce the GI disturbances associated with traditional iron supplements23. Novel formulations, including spherical lipid-coated particles, microspheres, nanoparticles, SLNs, liposomes, and sucrosomial iron (Table 2) have been developed to reduce GI side effects. The encapsulated iron helps to improve bioavailability based on higher solubility and permeability due to the lipoidal outer surface, bypassing carrier-mediated absorption33.This characteristic makes nanoparticles particularly suitable for children with ASD, who often experience heightened GI sensitivities.
| Type of Nanoparticles | Synthesis Method | Size range | Properties |
| Carbon-based NPs | co-precipitation | 1–100 nm | Electrical conductivity, high strength, structure, electron affinity, and adaptability |
| Zinc nanoparticles (ZnONPs) | combustion methods, ultrasound, microwave-assisted combustion method, two-step mechanochemical-thermal synthesis, anodization, co-precipitation, | 1–100 nm | Atalytic, electrical, optoelectronic, and photochemical capabilities |
| Copper nanoparticles (CuNPs) | top-down EEW approach | 1–100 nm | Antibacterial effects against various pathogens and antifungal activities |
| Iron nanoparticles (FeNPs) | Co-precipitation, Flame spray pyrolysis | 1−100 nm | Low toxicity |
| Lipid-based NPs | ultrasound, co-precipitation | 10–1,000 nm | Include lipid moieties |
Nanoparticles’ reduced size provides improved solubility
Nanoparticles have a size ranging between 1 and 100 nm. Such a dimension range implies higher solubility and tolerance with fewer side effects, making it an excellent condition for delivering iron to ASD children. A few of the recent studies relating to applying nanoparticles for site-specific drug delivery in the GI tract, thus enabling drug administration without compromising the effectiveness of other treatments, owing to adverse side effects18,34. The high specificity and solubility of nanoparticles have thus resulted in the reduced dosage required. Besides this, it ensures the stabilization of the compound, which is much more effective than the commercially available one in its pill form18. Other area considerations into nanoparticle enhancement include vitamin D and folic acid, whose specific findings regarding their potential to improve nutrient bioavailability in malabsorption syndromes have been established. Thus, this adds to the possible areas of interest related to iron supplementation35. Recent nanotechnology-based drug delivery systems, thus, hold both targeted and sustained release, which would overall minimize possible adverse effects since site-specific delivery within the body has already been attained19.
Nanoparticles improve iron delivery better than conventional treatment options
Studies are using different types of nanoparticles to validate the effectiveness of them in the iron delivery system. The design of the experiments and the key results of each study are summarized to show the consistent effectiveness and compare the ability of nanoparticles in improving IDA (Table 3).
An in vivo study conducted by Elshemy et al. compares the use of iron oxide nanoparticles and ferrous sulfate in the treatment of IDA in rats. By dividing 30 anemic rats into a control group, a ferrous sulfate group, and an iron oxide nanoparticles group with a 0.4 mg/kg dose for 10 days, it showed that iron oxide nanoparticles induced a significant increase in Red Blood Cell (RBC) count and hemoglobin concentration36 (Figure 1. A). The concentration of RBCs (106/μL) increased 27.1%, Hb (g/dL) increased 48.8%, and Hct increased 42.7%. On the other hand, Ferrous sulfate induced a RBC increase of 10.2%, Hb 36.5%, and Hct 31.4%. Moreover, there was a significant increase in serum ferritin, transferrin saturation, and serum iron levels in the iron oxide nanoparticles and ferrous sulfate groups when compared to the control group. Additionally, there was a significant decrease in C-reactive protein and serum MDA level in the iron oxide nanoparticles group when compared to the anemia group, which indicates the reduction of acute inflammation and oxidative stress, showing the potential of iron oxide nanoparticles in solving IDA as an anti-inflammatory substance.
Another study by F. Hashem et al. compares the efficacy of commercial iron products, uncoated iron oxide nanoparticles, and folic acid-coated iron oxide nanoparticles on treating iron deficiency37. For Mean blood Hb concentrations before and after 4 w of treatment with a dose of 2 mg/kg, commercial iron product shows a change of mean blood hemoglobin concentration increased by 118.95% (g/dl±SD, n=4). For uncoated iron oxide nanoparticles, it increased by 163%. Folic acid-coated iron oxide nanoparticles change by 294.5%. With a dose of 4 mg/kg, Commercial iron products increased by 178.87%, uncoated iron oxide nanoparticles increased by 206 %, and folic acid-coated iron oxide nanoparticles increased by 297.02% (Figure 1. B). The higher efficiency of iron oxide nanoparticles shown in the data is potentially caused by the enhanced bioavailability by increasing the surface area, which matches the research showing that nanosized particles can improve the cellular uptake and oral bioavailability.
Moreover, Helmy et al. compared the nanosized magnetite capped with a vitamin mixture with Ferric Chloride to anemic rats in 7-day treatments38. Iron oxides nanoparticles are capped with a mixture of multivitamins such as folic acid, Nicotinic acid (vitamin B9) and Ascorbic acid (vitamin C). They found that a small single dose of iron oxides-multivitamin nano-composite as low as 25 mg elemental iron per dose is sufficient to increase the hemoglobin level 232% within only four days after administration, and 100mg per dose can increase by 227% compared to the FeCl3 180% in 7 days (Figure 1. C). Additionally, there are no signs of toxicity in organs such as the liver, brain, spleen, kidney, and duodenum.
Iron solid lipid nanoparticles (Fe-SLNs) have also been studied by Hosney et al.39. 12 albino male rabbits weighing between 2 kg-2.5 kg were treated with 10mg/kg of ferrous sulfate tablet (control), and Fe-SLNs formulation and observed for 24 hours. Through an in vivo pharmacokinetic study, found that there was a statistically significant difference in the maximum plasma concentration and time required to reach maximum plasma level between the Fe-SLNs(330.51±15.18ng/mL and 0.75±0.3h) and the marketed tablet (90.22±8.21ng/mL and 2.0±0.2h), which indicated that using Fe-SLN increased the bioavailability by more than fourfold compared to Iron marketed tablet.
In a study examining iron solid lipid nanoparticles, after anemia induction, rats were treated with FeSO4, iron oxide magnetic nanoparticles-loaded liposomes (LMNPs), and Iron oxide magnetic nanoparticles (MNPs) with a dose of iron at 12 mg/kg of body weight for 13 days. Hb values of the control group, FeSO4, MNPs and LMNPs groups after anemia induction were 7.05, 7.15, 7, and 7.3 g/dl, respectively40. Hb value from LMNPs increased by 77.1%, which was significantly different from FeSO4 (40.8%), MNPs (39.0%), and control groups (18.4%). For RBCs concentration, after induction of anemia, the RBC count significantly decreased for all groups (4.1, 3.9, and 4.18 (million/cmm) for LMNPs, MNPs, and FeSO4, respectively. Thirteen days after treatment, the concentration of RBCs in LMNPs increased 68.7%, which indicated a remarkable increase in their RBCs count over the MNPs group (48.7%) and FeSO4 group (53.1%). The result of this study matches well with those of previous studies, which confirmed that naturally synthesized liposomal carriers increased the bioavailability of different drug molecules, resulting in higher bioavailability of LMNPs over FeSO4 and iron oxide magnetic nanoparticles. Additionally, the results of RBCs portray the benefits of liposomes. They protect the body from iron toxicity and prevent iron interaction with the surroundings in the gastrointestinal tract, demonstrating their ability to reduce the side effects of the supplementation on GI issues.
In a study evaluating zinc oxide particles, participants were allocated to chronic(1 month) or acute(24 hours) treatment groups groups with 0.3 mL suspension of nanoparticles (10 mg/mL)41. The acute group’s results show that RBC count and Hb significantly increased in two Fe3+,2+ groups at 24 h and one week after nanoparticle application. In the chronic application of Fe3+,2+, ZnO nanostructures were the most effective in increasing ferritin levels and improving hematological parameters, with no influence one hepcidin expression. The level of hemoglobin was significantly increased in almost all experimental groups. The study found that iron-doped ZnO nanoparticles improved hematological parameters efficiently, such as hemoglobin, RBC, and hematocrit, in hematopoietic tissues without inducing toxic side effects or altering hepcidin expression. The nanoparticles were found to be biodegradable, with gradual release of iron ions, which can mitigate some toxic effects of iron-doped substances. However, the study pointed out the potential impact of chronic application of iron oxide nanoparticles on neurological and physiological disturbances. This indicated the concerns of the long-term impact of nanoparticles as oral supplements.
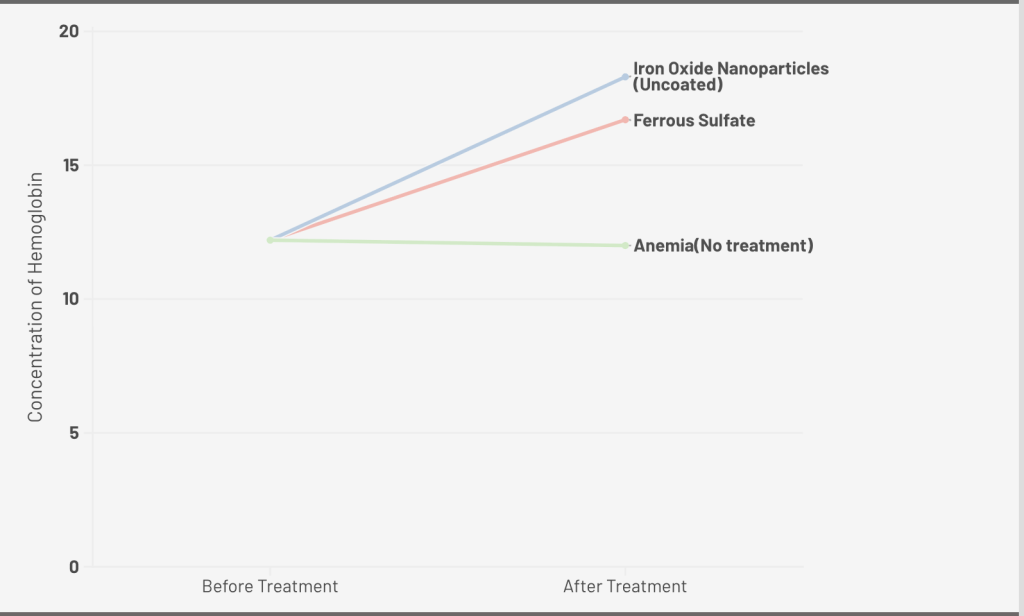
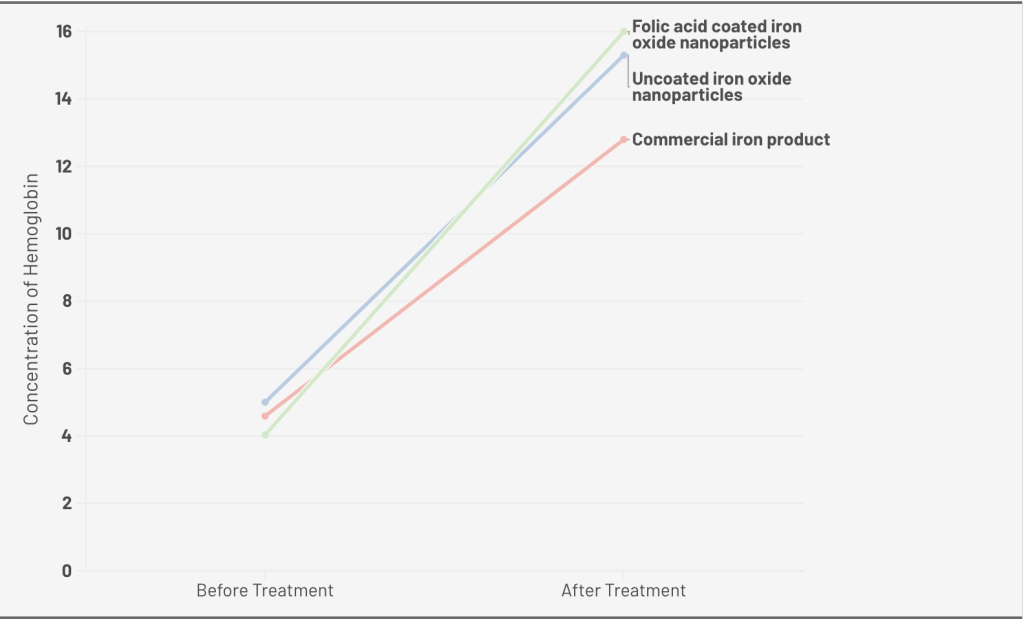

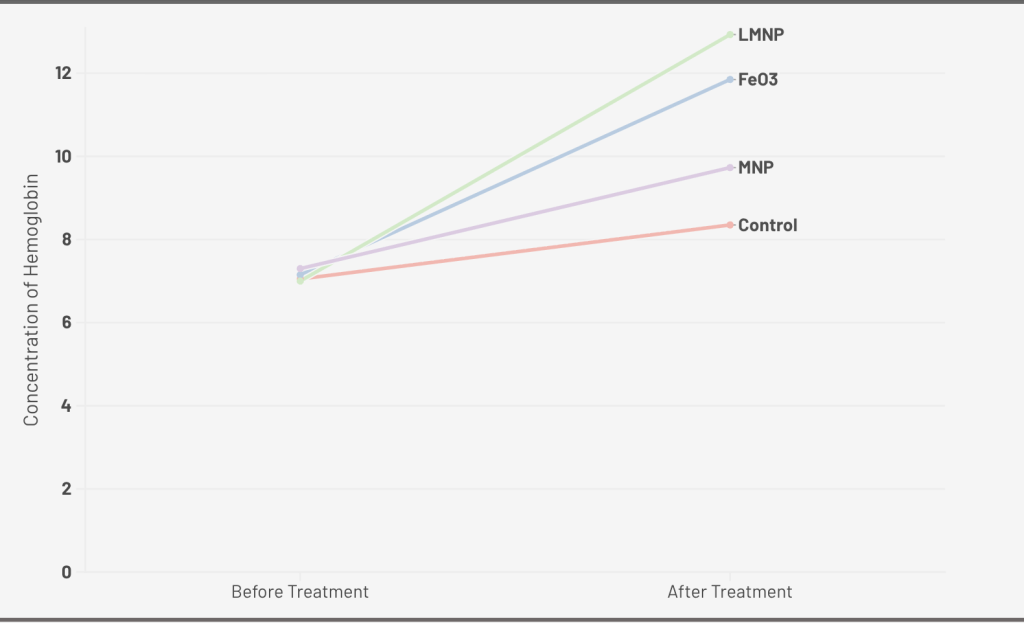
A. 0.4 mg/kg dose of Iron Oxide Nanoparticle (Uncoated), Ferrous Sulfate for 10 days; B. 2 mg/kg dose of Folic Acid Coated Iron Oxide Nanoparticle, Iron Oxide Nanoparticle (Uncoated), Commercial Iron Product for 4 weeks; C. 100mg/kg does of Nano-sized Magnetite capped with Vitamins, FeCl3, and Vitamin Mix(Control) for 7days; D. 12 mg/kg dose of Iron Oxide Magnetic Nanoparticles-Loaded Liposome, Iron Oxide Magnetic Nanoparticles, FeSO4, and control for13 days.
| Nanoparticles | Studies | Dose | Animal Used | Hemoglobin (Hb) | Unique Parameter | |
| Iron Oxide Nanoparticles (Uncoated) | Elshemy et al. | iron oxide nanoparticles 0.4 mg/kg b.w/ 10 days (drinking water). | Forty male albino rats with an average body weight of 125 g were used. | 48.8% (18.46±0.33 g/dL) | A significant decrease in C-reactive protein and serum MDA level | |
| Iron Oxide Nanoparticles (Folic Acid-Coated) | F. Hashem et al. | 4 w of treatment with a dose of 2 mg/kg; 4 w of treatment with a dose of 4 mg/kg | Thirty-two female Wistar rats (3 weeks old), weighing 100±10 g | 294.5% (15.9±0.57; 16.0 ± 0.71 g/dL ) | Indicate higher Hb concentration verses uncoated IONPs at the same dose | |
| Nano-Sized Magnetite | Helmey et al. | 100mg/kg | Five weeks old Albino rats of the Wisconsin Holtzman strain, weighing 150-160g | 227% (14.6g/dL) | The hemoglobin concentration of the magnetite-treated group exceeded all other groups, including the non-anemic one | |
| Iron Solid Lipid Nanoparticles (Fe-SLNs) | Hosny et al. | 10 mg/kg | Twelve albino male rabbits, weighing between 2 kg and 2.5 kg | Increased the bioavailability by more than fourfold compared to Iron marketed tablet. | ||
| Iron Oxide Magnetic NPs-Loaded Liposomes (LMNPs) | Fathy et al. | 12 mg/kg of body weight (13days) | Thirty-five adult female Wistar rats weighing 100-120g | 77.1% (12.93 g/dL ) | The histopathological structures of the liver, spleen and kidney remain normal. | |
| Iron oxide magnetic nanoparticles (MNP) | Fathy et al. | 12 mg/kg of body weight (13days) | Thirty-five adult female Wistar rats weighing 100-120g | 39.0% (9.73 g/dL) | ||
| Zinc Oxide NPs (Fe-doped) | Kielbik et al. | Adult Mice | Significantly increased. The chronic application of Fe3+,2+ doped ZnO nanostructures was the most effective | No effect on hepcidin; improves ferritin with chronic application |
Discussion
Iron deficiency anemia (IDA) was highly prevalent in the children with ASD, with prevalence ranging from approximately 15.6% to 52% in studies focusing on iron status in various regions. Several studies note possible contributing factors, including patient food preferences, leading to restricted diets, and the prevalence of GI disturbances that result in poor iron absorption, which may make eating iron-containing foods challenging. Several studies hypothesize that there may be differences in enzyme activity levels, such as GABA-aminotransferase and dopamine, whose function could be compromised by iron deficiency. In children with ASD, however, determining the pathology of poor iron absorption in ASD would require studies using advanced biomarkers of iron metabolism and absorption in children with ASD.
Historically, studies have assumed that ASD was causative of low serum ferritin. However, some studies have also hypothesized that, conversely, low serum ferritin may be a contributing factor to the development of ASD8. Supportively, it has been documented that children with ID and IDA have a high incidence of disturbed brain development, which could point to malabsorption syndrome and poor neurodevelopment. This discussion of potential causation is of particular importance because if, in fact, low serum ferritin is contributing to the development of ASD, then addressing IDA in children with GI disorders may be of greater importance than previously thought42, underscoring the importance of studies addressing alternative methods of iron supplementation in this population.
Iron supplementation was again noted to be associated with improved sleep behavior among children with autism and sleep disorders, hence possibly linking iron deficiency and sleep disorders among children with autism spectrum disorder. Such deep-rooted and widespread consequences of iron deficiency on development and health have led to IDA treatment being one of the main components in their therapy for these children. However, studies have shown that conventional iron supplements do not take the specific needs arising from such a condition into account because it may exacerbate GI issues, which is particularly important for children with ASD who might already experience gastrointestinal problems. Although there are no studies that examine this specifically in ASD, ASD has a high burden of GI symptoms. There are studies show that nanoparticles can minimize GI issues and malabsorption, so they have a great potential to be useful in this population.
This kind of delivery would have reduced the most challenging systemic adverse effects in this highly sensitive group. So far, changes in several parameters have indicated that nanoparticle systems will be invaluable in iron delivery, with various in vivo studies demonstrating this. Reduced dosage and perhaps a more favorable profile of possible side effects may ensure better compliance and a more pleasant experience in ASD children. Nanoparticles appear to be a potential solution. However, the extensive usage of NPs may cause oxidative stress and lead to health issues43, and some metal oxide nanoparticles also have the risk of accumulating in nucleus where they damage the DNA44, which might lead to concerns about their bio-toxicity and long-term safety issues.
Through the animal experiments, all the nanoparticles show a significant improvement in iron parameters, indicating their ability to mitigate IDA with high efficiency. Iron oxide NPs are usually very cost-effective to obtain nanoparticles, and their controlled shape and small sizes allow them to interact and bind with metal ions efficiently45. However, various studies have reported probable accumulation of these NPs in spleen and kidney46. Specifically, ingesting it might lead to harmful side effects to organs, such as lung cancer47, which might lead to some safety issues on the long-term usage of these NPs.
Iron solid lipid nanoparticles usually perform better bio-compatibility and good entrapment efficiency48. The existence of lipid in SLN is effective in preventing acute and chronic toxicity49. Additionally, the form of solid lipid enables its slow-down of lipid digestion, therefore leading to a sustained release, improving the stability, and increasing the absorption after incorporation into solid lipid nanoparticles. Under the same situation, folic acid-coated iron oxide shows greater ability than uncoated iron oxide nanoparticles in improving the Hb concetration. This phenomenon might be explained that the folic acid coating acts as a stabilizer to prevent the aggregation and or reaction with other components in the gastrointestinal tract, which enhance the solubility of iron core. On the other hand, the applications of SLN still exists several concerns. The traits of SLN might sometimes result in unpredictable gelation tendency49, leading to the failure or instability of drug absorption for children with autism.
Furthermore, for other metal-doped iron oxide nanoparticles, their smaller sizes tend to exhibit higher absorption efficiency, and their simplicity and large-scale synthesis lead to a wide range of applications in different fields50. However, there exists toxicity that is related to ZnO-NPs, including the oxidative stress and inflammation induced by the accumulation of zinc ions51, which might induced damage to the brain has a strong potential to negatively impact normal CNS functions52. The existence of oxidative stress might make it unsuitable for children with autism, who are extremely sensitive toward oxidative stress, and it might has the potential of harming the gut microbiota that can further exaggerate the ASD symptoms.
Overall, while these studies underscores all available evidence on the prevalence of ID/IDA on children with ASD, the severe risks of it that impacts on these children’s development, and the in vivo potential use of nanoparticles, it includes several limitations: There are limited experimental studies with consistent outcomes that can be compared across studies, only animal studies are available to answer the questions on nanoparticles, which may not be directly translatable to this topic in humans, and some of the studies have some small sample size that may not lead to reliable outcomes.
Even so, this literature review effectively set the stage for future experimental studies on nanoparticles would be a possible solution concerning the delivery of iron among the autistic population intolerant of conventional supplements, specifically including the experimental design that compares the efficacy of traditional treatment of IDA to nanoparticle treatment. Therefore, nanoparticles would be a possible solution concerning the delivery of iron among the autistic population intolerant of conventional supplements.
However, there is no paper that links iron nutrient delivery via nanoparticles to autism, which has potentially left a number of safety concerns regarding such a delivery system. This finding indicates the need for further investigation about the use of nanoparticles targeting the autistic children, and creates more possibilities for this special group.
Conclusion
Thus, this review has underlined the current state of iron deficiency in children with autism, pointing out the existing limitations of commercially available iron supplements by explaining gastrointestinal sensitivity and malabsorption issue, which demands new delivery systems for iron. Iron deficiency anemia in children with ASD might be managed based on the potential and properties of nanoparticles for increased solubility, targeting, and bioavailability. In vivo studies on nanoparticles, such as iron oxide, solid lipid nanoparticles, and zinc oxide, have shown the results of improvement in different parameters and iron stores with potentially reduced side effects compared to conventional iron supplements.
However, these would need considerable further investigation into their safety and efficacy in the ASD community. A longitudinal study may be needed to substantiate the intervention’s long-term effect on hemoglobin levels, further indicate the nanoparticles’ role in promoting iron absorption, and reducing the gastrointestinal disturbance in the autistic population.
Hence, there could be good potential for the iron delivery nanoparticles in treating IDA in children with ASD, proceeding into clinical practice and ensuring a better quality of life for these special populations.
Acknowledgements
Thanks to the people who support the study.
References
- American Psychiatric Association. Autism Spectrum Disorder. https://www.psychiatry. org/patients-families/autism (2019). [↩]
- M. D. Kogan, C. J. Vladutiu, L. A. Schieve, R. M. Ghandour, S. J. Blumberg, B. Zablotsky, J. M. Perrin, P. Shattuck, K. A. Kuhlthau, R. L. Harwood, M. C. Lu. The prevalence of parent-reported autism spectrum disorder among US children. Pediatrics 142 (2018). [↩]
- J. Zeidan, E. Fombonne, J. Scorah, A. Ibrahim, M. S. Durkin, S. Saxena, A. Yusuf, A. Shih, M. Elsabbagh. Global prevalence of autism: a systematic review update. Autism Research 15, 778-790 (2022). [↩]
- J. L. Beard, J. R. Connor. Iron status and neural functioning. Annual review of nutrition, 23 (2003). [↩]
- B. Lozoff, J. Beard, J. Connor, B. Felt, M. Georgieff, T. Schallert. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutrition Reviews 64 (2006). [↩] [↩]
- J. Kim, M. Wessling-Resnick. Iron and mechanisms of emotional behavior. The Journal of Nutritional Biochemistry 25 (2014). [↩]
- A. Latif, P. Heinz, R. Cook. Iron deficiency in autism and Asperger syndrome. Autism 6, 103-114 (2002). [↩] [↩] [↩] [↩]
- S. Gunes, O. Ekinci,T. Celik. Iron deficiency parameters in autism spectrum disorder: clinical correlates and associated factors. Italian Journal of Pediatrics 43 (2017). [↩] [↩] [↩]
- S. Sidrak, T. Yoong, S. Woolfenden. Iron deficiency in children with global developmental delay and autism spectrum disorder. Journal of Pediatrics and Child Health 50 (2013). [↩]
- S. Hergüner, F. M. Keleşoğlu, C.Tanıdır, M. Çöpür (2011). Ferritin and iron levels in children with autistic disorder. European Journal of Pediatrics 171 (2011). [↩]
- C. F. Dosman, J. A. Brian Drmic, I. E. Drmic, A. Senthilselvan, M. M. Harford, R. W. Smith, W. Sharieff, S. H. Zlotkin, H. Moldofsky, S. W. Roberts. Children with Autism: effect of iron supplementation on sleep and ferritin. Pediatrics Neurology,36 (2007). [↩]
- S. F. Al-Ali, A. Alkaissi. Association between autism spectrum disorder and iron deficiency in children diagnosed autism spectrum disorder in the Northern West Bank. J Health Med Nur, 16 (2015). [↩]
- M. Al-Beltagi, N. K. Saeed, A. S. Bediwy, R. Elbeltagi, R. Alhawamdeh. Role of gastrointestinal health in managing children with autism spectrum disorder. World Journal of Clinical Pediatrics 12, 171-196(2023). [↩] [↩] [↩]
- M. Bresnahan, M. Hornig, AF. Schultz, N.Gunnes, D. Hirtz, KK. Lie, P. Magnus, T. Reichborn-Kjennerud, C. Roth, S. Schjølberg, C. Stoltenberg, P. Surén, E. Susser, WI. Lipkin. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry, 72 (2015). [↩]
- S. R. Bloor, R. Schutte, A. R. Hobson. Oral Iron Supplementation—Gastrointestinal Side Effects and the Impact on the Gut Microbiota. Microbiology Research, 12 (2021). [↩] [↩]
- I. Khan, K. Saeed, I. Khan. Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry 12, 903-931(2017). [↩]
- M. Chudasama, J. Goyary. Nanostructured materials in food science: Current progress and future prospects. Next Materials 5, 100206(2024). [↩]
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. del P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy. [↩] [↩] [↩]
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas, R. Langer. Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery 20, 1–24 (2020). [↩] [↩]
- K. Singh, D. C. Sethi, D. Singh, N. Singh. Nano-formulations in treatment of iron deficiency anaemia: An overview. Clin Nutr ESPEN (2022). [↩]
- A. Edgar, Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Current Opinion in Colloid & Interface Science 14, 3-15 (2008). [↩]
- P. K. Saha, L. Saha. Iron nanoparticles and its potential application: A literature review. Indian Journal of Pharmacology 53, 339 (2021). [↩]
- A. Kumari, A. K. Chauhan. Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: a review. Journal of Food Science and Technology 59, 3319-3335(2022). [↩] [↩] [↩]
- A. Shukla, N. Dasgupta, S. Ranjan, S. Singh, R. Chidambram. Nanotechnology towards prevention of anaemia and osteoporosis: from concept to market. Biotechnology & Biotechnological Equipment 31, 863-879 (2017). [↩] [↩]
- R. C. Carter, J. L. Jacobson, M. J. Burden, R. Armony-Sivan, N. C. Dodge, M. L. Angelilli, B. Lozoff, S. W. Jacobson. Iron deficiency anemia and cognitive function in infancy. Pediatrics 126, 427-434 (2010). [↩]
- S. Ranjan, JA. Nasser. Nutritional status of individuals with autism spectrum disorders: do we know enough? Adv Nutr (2015). [↩]
- A. C. Fernandez-Gaxiola, L. M. De-Regil. Intermittent iron supplementation for reducing anemia and its associated impairments in adolescent and adult menstruating women. Cochrane Database of Systematic Reviews (2019). [↩]
- RJ. Stoltzfus, ML. Dreyfuss. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. INACG (1998). [↩]
- T. G. DeLoughery. Safety of Oral and Intravenous Iron. Acta Haematologica 142, 8-12 (2019). [↩]
- Z. Tolkien, L. Stecher, A. P. Mander, D. I. A. Pereira, J. J. Powell. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLOS ONE 10 (2015). [↩] [↩]
- T. Buie, D. B. Campbell, G. J. Fuchs, G. T. Furuta, J. Levy, J. VandeWater, A. H. Whitaker, D. Atkins, M. L. Bauman, A. L. Beaudet, E. G. Carr, M. D. Gershon, S. L. Hyman, P. Jirapinyo, H. Jyonouchi, K. Kooros, R. Kushak, P. Levitt, S. E. Levy, J. D. Lewis. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics 125, S1-S18(2010). [↩]
- M. J. Maenner, C. L. Arneson, S. E. Levy, R. S. Kirby, J. S. Nicholas, M. S. Durkin. Brief report: Association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. Journal of Autism and Developmental Disorders 42, 1520–1525 (2012). [↩]
- S. Trivedi, S. Shah, R. Patel. Review on novel oral iron formulations with enhanced bioavailability for the treatment of iron deficiency. Journal of Drug Delivery Science and Technology 90, 105181 (2023). [↩]
- H. Laroui, D. S. Wilson, G. Dalmasso, K. Salaita, N. Murthy, S. V. Sitaraman, D. Merlin. Nanomedicine in GI. American Journal of Physiology-Gastrointestinal and Liver Physiology 300, 371–383 (2011). [↩]
- R. Arshad, L. Gulshad, I. Haq, M. A. Farooq, A. Al‐Farga, R. Siddique, M. F. Manzoor, E. Karrar. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Science & Nutrition 9, 3354–3361 (2021). [↩]
- M. Elshemy. Iron oxide nanoparticles versus ferrous sulfate in treatment of iron deficiency anemia in rats. Egyptian Journal of Veterinary Sciences 49, 103–109 (2018). [↩]
- F. Hashem, M. Nasr, Y. Ahmed. Preparation and evaluation of iron oxide nanoparticles for treatment of iron deficiency anemia. International Journal of Pharmacy and Pharmaceutical Sciences, 10, 142 (2018). [↩]
- S. H. A. Helmy, M. B. M. Mahmoud. Novel formula of iron based nanocomposites for rapid and efficient treatment of iron deficiency anemia.https:// patents.google.com /patent/WO2014135170A1/en (2013). [↩]
- K. Hosny, Z. Banjar, A. Hariri, A. H. Hassan. Solid lipid nanoparticles loaded with iron to overcome barriers for treatment of iron deficiency anemia. Drug Design, Development and Therapy 313 (2015). [↩]
- M. M. Fathy, H. M. Fahmy, A. M. Balah, F. F. Mohamed, W. M. Elshemey. Magnetic nanoparticles-loaded liposomes as a novel treatment agent for iron deficiency anemia: In vivo study. Life Sciences 234, 116787, (2019). [↩]
- P. Kiełbik, A. Jończy, J. Kaszewski, M. Gralak, J. Rosowska, R. Sapierzyński, B. Witkowski, Ł. Wachnicki, , K. Lawniczak-JablonskaPiotr Kuzmiuk, P. Lipiński, M. Godlewski, M. M. Godlewski. Biodegradable zinc oxide nanoparticles doped with iron as carriers of exogenous iron in the living organism. Pharmaceuticals 14, 859–859 (2021). [↩]
- Z. Tolkien, L. Stecher, AP. Mander, D. I. Pereira, JJ. Powell. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLOS One (2015). [↩]
- J. K.Tee, C. N. Ong, B. H. Bay, H. K. Ho, D.T. Leong. Oxidative stress by inorganic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 8 (2016). [↩]
- M. Horie, K.Fujita. Toxicity of metal oxides nanoparticles. Elsevier 5 (2011). [↩]
- A. Ali, H. Zafar, M. Zia, I. ul Haq, A. R. Phull, J. S. Ali, & A. Hussain (2016). Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnology, Science and Applications 9 (2016). [↩]
- D. Edge, C. M. Shortt, O. L. Gobbo, S. Teughels, A. Prina-Mello, Y. Volkov, P. MacEneaney, M. W. Radomski, F. Markos. Pharmacokinetics and bio-distribution of novel super paramagnetic iron oxide nanoparticles (SPIONs) in the anaesthetized pig. Clinical and Experimental Pharmacology and Physiology 43 (2016). [↩]
- R. D. Handy, B. J. Shaw. Toxic effects of nanoparticles and nanomaterials: Implications for public health, risk assessment and the public perception of nanotechnology. Health, Risk & Society 9 (2007). [↩]
- S. Bhatia. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. Springer, Cham (2016). [↩]
- S. D. Mandawgade, V. B. Patravale. Development of SLNs from natural lipids: application to topical delivery of tretinoin. Int J Pharm 363 (2008). [↩] [↩]
- U. K. S. Raghavendra, R. Annamalai, G. Induja, N. Anup,The purview of doped nanoparticles: Insights into their biomedical applications. OpenNano 8 (2022). [↩]
- R. Solano, D. Patiño-Ruiz, L. Tejeda-Benitez. et al. Metal- and metal/oxide-based engineered nanoparticles and nanostructures: a review on the applications, nanotoxicological effects, and risk control strategies. Environ Sci Pollut Res 28 (2021). [↩]
- S. Salim. Oxidative Stress and the Central Nervous System. J Pharmacol Exp 360 (2017). [↩]