Abstract
This literature review examines the impact of chronic stress on neuroplastic connections and memory retention in adolescents. Findings have been synthesized from neuroscientific and behavioural studies across age groups, cultures, samples and genders. Chronic stress disrupts brain functions due to elevated cortisol levels, BDNF levels, impairing synaptic plasticity by reducing neuroplastic connections particularly in the hippocampus and prefrontal cortex. These factors lead to depleted memory which weakens learning ability, ultimately decreasing academic performance. This review also explores the role of other factors such as sleep quality, social support, socioeconomic status and exercise on improving neural connections in the brain.
Neuroplasticity
Neuroplasticity refers to the brain’s ability to adapt its structure and function in response to environmental stimuli, learning, or injury by forming or reorganising neural networks. These connections can be changed in response to a changing environment, injury, learning, development, or experiences. Neural functions flex and adapt to develop according to localized regions in the brain; this helps in the organisation and modification of information. In 1891, a French surgeon discovered a region called “Broca’s area”1, in the left hemisphere of the brain, which contains motor neurons solely responsible for the articulation of speech. When a synapse in the brain is repeatedly activated, neurons grow and form stronger connections, enhancing neural plasticity. This explains why regular practice of an activity leads to improved performance. Meanwhile, unused synapses are gradually eliminated, breaking old neural connections and creating space for new information. Although this can result in forgetting details, when the brain attempts to compensate for this lost information, it can cause physical damage (e.g., stroke or neurological problems) (Maeshima and Osawa).
There are two types of neuroplasticity: structural and functional. Structural neuroplasticity is when the brain’s physical tissue changes. For example, when one learns a new language, especially in adulthood, parts of the brain like Broca’s and Wernicke’s areas become thicker as neural networks in these parts build and get denser, allowing learning, remembering, and processing of a new language. Functional neuroplasticity is when new synaptic connections are formed by neurons to compensate for damage by moving functions to undamaged areas. When a patient is recovering from traumatic brain injuries (like strokes), other parts of the brain take over the functions of the damaged parts, allowing repair of neurons.
Effect of age on synaptic density
Synaptic density, the number of synapses in a given area of the brain, generally remains relatively stable throughout an adult’s life. Our brains can develop synaptic connections with age. Synaptic connections rapidly increase and maximize around infancy(age 1-2)2. During adolescence, the brain undergoes neural pruning. In this process, unused or weak synaptic connections are removed. This process strengthens frequently used neural pathways, enhancing the brain’s efficiency in learning and memory.
The Huttenlocher plot shows synaptic density in layer 3 of the cortex versus age3. It suggests that synaptic density decreases with age, causing reduced function in cognition because a loss in synaptic junctions disrupts the brain’s ability to store, remember, and process information.

Brain Derived Neurotrophic Factor (BDNF)
Brain-derived neurotrophic factor is a molecule that belongs to neurotrophins; it is assumed to be essential for survival, differentiation of neurons, and increased neuroplasticity5. This neuroprotective factor (substances that prevent the nervous system from degradation) improves synaptic plasticity, enabling the formation of long-term memory and the functioning of other cognitive functions. Neurotrophic factors can cross the blood-brain barrier, which causes the BDNF concentration to move from higher levels to lower levels into the peripheral tissue; therefore, it can be used as a justified biomarker for finding correlations.
Adolescence is considered to be the most crucial period for brain maturation and plasticity; hence, BDNF levels usually show an increase compared to younger children. Maintaining habits like exercising and consumption of healthy food is extremely necessary, as it controls the amount of BDNF produced and released. Exercise increases blood flow, allowing a higher concentration of oxygen and nutrients to be transported to the brain, which promotes neuronal growth and survival of synapses. Physical activity stimulates muscular activity, which promotes the release of factors like Cathepsin B, which further enhances BDNF release and cognitive function.
A study carried out by Marlatt et al. (2012)6, aimed to assess how exercise (voluntary running) enhances BDNF gene expression in mice (aged 4 weeks). The mice were divided into two groups: control and exercise. Each mouse in the exercise group was provided with a running wheel, whereas the control group was not. The running distance and time were monitored for 30 days. The animals were sacrificed, and then tissue layers were collected for examination. The results showed that volunteer running significantly induced BDNF promoter I and II expression in the hippocampal region of the brain, thus concluding that exercise significantly affects stress and BDNF levels in one’s brain.
While this study provides a link between exercise and increased BDNF expression, the extent to which animal models are compared to human models is questionable. Animal research has notable limitations, as it relies on a comparative approach, which assumes that humans and animals share many biological mechanisms, such as neural structure and function. The generalizability of these studies is very low, as the complexity of the brain, hormone functions, social behaviour and environmental factors greatly vary. Animal models cannot replicate the human neurobiological behaviour, which limits the construct validity of these findings.
On the other hand, downregulation of BDNF levels can also occur when there is chronic intake of alcohol. This causes BDNF levels to decrease in the brain and blood, which causes neurocognitive deficits and impairments in the body. Ethanol vapors diffuse throughout the organs in the body via the bloodstream and disrupt the BDNF-TrkB signaling pathway. Firstly the BDNF binds to a specific Tropomyosin receptor kinase B (TrkB) receptor, which is present on the surface of the neuron. This binding triggers the dimerization (condensation) of TrkB receptors, which decreases the gap between intercellular TrkB domains, allowing autophosphorylation. This activates the receptor’s tyrosine kinase activity initiating the reaction between multiple intracellular pathways.
First is the MAPK (Mitogen-activated protein kinase) pathway which is involved in cell growth, differentiation and survival. The second pathway is the PI3K (Phosphatidylinositol 3-kinase) pathway, which is in charge of cell metabolism. Lastly the LC-γ (Phospholipase C-gamma) pathway, involved in calcium signalling and maintaining synaptic plasticity. The activation of these major pathways under the exposure of ethanol cause a chain reaction of downstream effects which alter gene and neural functioning. Additionally, this reduces the effectiveness of TrkB (BDNF receptors) to transmit signals, causing impaired neuroplasticity, reducing synaptic connections in the brain, and ultimately affecting memory and recall, along with changes in mood and increased vulnerability to neurodegenerative diseases.

Other relevant neurotrophins involved in stress and neuroplasticity
Although BDNF is one of the most studied neurotrophins, other neurotrophin molecules like nerve growth factor (NGF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4) also play a crucial role in stress and neuroplasticity.
Nerve growth factor (NGF) is a protein that plays its principal role during development in adulthood, ensuring the regulation of phenotypic and functional characters of a population of neurons in the peripheral nervous system (PNS) and central nervous system (CNS) (Aloe et al). It is a type of neurotrophic factor that maintains and supports the health and function of nerve cells involved in the development of sympathetic and sensory neurons, along with guiding the growth of axons (nerve cell extensions). Chronic stress leads to the production of antibodies which attack healthy NGF, interfering with its normal function. Reduced NGF levels potentially stop the brain’s ability to regulate mood and anxiety, which may contribute to neurodegeneration and stress-related brain disorders. (Eckart et al., 2013)
Neurotrophin-3 supports the ability of a neuron in the CNS to differentiate and survive by binding to the TrkC receptor. It is involved in several processes like axon growth, synapse formation and synaptic plasticity, which are essential for forming neural connections and storing information in the brain (Neurotrophin-3 modulates synaptic transmission). The expression of NT-3 starts at birth and continues throughout one’s lifetime, particularly to help hippocampal neurogenesis. Hence, when NT-3 levels in the brain decrease due to chronic stress, the ability to strengthen and maintain the efficiency of neural pathways becomes challenging, resulting in reduced memory retention.
Effect of stress on BDNF regulation
Stress exposure can trigger changes in BDNF expression, thus decreasing its levels and lowering the efficiency of cognitive functioning, although factors such as environment, timing, type of stress and age also matter. The HPA axis, through the release of cortisol, regulates stress response via a negative feedback loop in the brain (increased levels of cortisol released by adrenal glands signal the hypothalamus to decrease the release of cortisol, helping the body to return to homeostasis). However, prolonged stress can cause a longer exposure to high cortisol levels in the brain, leading to multiple impairments (e.g., reduced synaptic growth, memory loss, mood regulation) and dysfunctions, which hinder homeostatic balance, causing lower BDNF levels.
A 2016 study by María José Míguez, published in the Journal of Neurological Sciences and Psychiatry7, explored the impact of stressors like parental divorce, academic pressure, and migration on BDNF levels in Hispanic adolescents. This study included 450 participants whose BDNF and proBDNF levels were obtained at 3 points during the length of the study. The results concluded that even after the growth spurt of the brain during childhood, the brain size remained constant during adolescent years due to many factors like persistent abnormal BDNF levels, environmental stressors, diet, and access to healthcare. Although there might be a potential link between these two factors, direct causal links between BDNF levels and brain volume cannot be drawn because there may be other biological factors influencing this change.
Firstly this study provides strong statistical evidence due to its large and focused sample size. Secondly, the BDNF and pro-BDNF levels were tracked over a long period of time, enabling researchers to collect in depth data on the long term effect of neurotrophins on adolescents. Lastly, the real world relevance of this study increases its ecological validity as it uses environmental stressors such as parental divorce, migration and separation. However, since this study collected data by the self report method, factors such as demand characteristics could have affected the participants’ responses. Furthermore, the lack of neuroimaging data also questions the extent to which the findings are biologically relevant and precise, as the brain function and structure remains unclear.

Neuroimmune and neuroendocrine interactions affecting neuroplasticity
Chronic stress can trigger the activation of a complex neuroimmune response which leads to dangerous health consequences like physical challenges in movement, immunity impairment, or psychological damage (Christina L. Graves a d et al., 2023). A neuroimmune dysfunction occurs when chronic stress changes the homeostasis of the body, due to prolonged activation of the HPA axis and release of proinflammatory cytokines like IL-1β, IL-6, and TNF-α. These cytokines play an important role in immunity, although their interaction with neural networks can contribute to inflammation in some areas of the brain like the hippocampus and prefrontal cortex, negatively impact synaptic plasticity and memory retention. The long term potentiation (LTP) is responsible for learning and remembering, although elevated inflammatory signalling (Wiley) can suppress this mechanism which puts cognitive functions at risk.
The neuroendocrine system bridges the gap between the nervous and endocrine system by regulating bodily functions through nerve and hormone producing cells (Canadian Cancer Society). It controls vital processes like reproduction, growth and metabolism while keeping homeostasis of stress levels in the body and regulating neural plasticity. Prolonged elevation in Corticotropin-releasing hormone (CRH) levels causes chronic stress due to the suppression of the BDNF gene expression in key areas of the brain. This happens as CRH is released by the hypothalamus, which subsequently secretes ACTH and cortisol from the adrenal cortex. Higher CRH levels lead to overproduction of cortisol which diminish the amount of critical neurotrophins present. The drop of these BDNF levels damages neural plasticity, synaptic strength, neurogenesis and dendritic remodelling (Yang et al., 2020).
Dendritic remodeling and synaptic pruning in regions of the brain affected by stress
Dendritic remodelling is the structural change that occurs in the dendrites of neurons, they either retract or extend which changes the dendritic spine density, altering the shape of the spine. This process is a core mechanism of neuroplasticity as it can either strengthen or weaken synaptic plasticity. If the body undergoes adaptive remodelling, which includes the ability to learn new information, synaptic connection strength, increasing synaptic plasticity. Although the body experiences maladaptive remodelling, which is when a synaptic connection formed produces aberrant or negative symptoms (Puderbaugh, 2023).
Chronic stress is one of the most prominent negative triggers which causes maladaptive remodelling (McEwen, 2017). The overactivation of the HPA axis affects various regions of the brain, primarily the hippocampus, amygdala and prefrontal cortex. CA1 and CA3 pyramidal neurons are types of neurons present in the hippocampus critical for retrieval of memory and memory encoding, respectively. Cortisol binds with glucocorticoid receptors (GRs), which are present in abundance on the membrane of the neurons. Although under chronic stress the elevated glucocorticoids levels lead to dendritic retraction and reduction in spine density, weakening the neural circuitry. This causes numerous deficits in learning and recall such as reduced spatial memory, reduced recall of episodic memory and reduced memory consolidation.
Additionally, the glucocorticoid mechanism also affects dendritic spine density in the prefrontal cortex, which is responsible for decision making and emotion regulation. Microglial activation is the process by which microglia (immune cells of the CNS) experience a sudden shift from a resting state to active state, in response to injury, stress, infection of environmental stimuli (Qin et al., 2023). The activation of microglial cells allows to maintain homeostasis and brain development by causing release of inflammatory mediators (neuroinflammation), synaptic pruning of unhealthy connections and phagocytosis of damaged cells in the brain. However during stressful situations, the elevated glucocorticoid mechanism reduced dendritic density in layer II/III pyramidal neurons of the medial prefrontal cortex. This dysregulation contributes to over-pruning as it weakens neural circuits as it kills healthy connections, weakening memory and executive functions.
Paolicelli et al. (2011) aimed to investigate whether microglia play an active role in synaptic pruning during brain development and how their dysfunction can affect neural circuits in different regions of the brain. The sample included wild mice with C3CR1 knockout gene. Researchers observed the microglial behaviour in the hippocampus during the prenatal development phase of the mice, by using confocal microscopy imaging. The synaptic density and microglial interactions were compared between the control and knockout gene groups. The results displayed that the normal mice microglia actively engulfed synaptic elements during brain maturation, however in the knockout gene mice (impaired microglia) there was a delay in synaptic pruning. This helped researchers conclude that microglia are essential for synaptic pruning during early development. Dysregulation of microglia can result in neurodevelopmental disorders such as impaired cognitive functions.
The application of advanced imaging techniques allow researchers to clearly compare the differences in microglial activation. Additionally the use of genetically different mice help researchers to form causal links between microglial-synapse interactions. However since this study was conducted on mice, the generalisation cannot be fully made to human neurobiology. This study also only focuses on early development without providing longitudinal effects during adolescence or adulthood. Lastly the lack of exploration in behaviour and cognition could also question the validity of this study.
Memory
Memory is the psychological process of acquiring, storing, and retaining information.8. Many parts of the brain work together to perform tasks like problem-solving, navigating through spaces, language development, and many more. Memories are formed by three major processes: encoding, storage, and retrieval. Encoding involves forming and growing synaptic chains that occur largely in the hippocampus region of the brain. Storage refers to how information is stored within the synaptic gaps, most notably in the prefrontal cortex, amygdala, and hippocampus. Retrieval is the process by which information is remembered and recalled. This involves the strengthening of neuronal pathways, which occurs via active recall and rehearsal of information.
Short-term memory, also referred to as active or primary memory, serves as the brain’s temporary storage system, holding sensory information for 5 to 18 seconds9. It is mainly used for taking in sensory information from the surroundings (like voices, smells, sights, and textures). However, long-term memory is nearly a permanent storage of learned information and memories. Memory consolidation is the process by which short-term memory is converted into long-term. This occurs when one rehearses information in spaced intervals repeatedly, which causes the structure of the synaptic gaps to become more stable and reliable, making it more resilient to deletion.
Effect of age on retention and accuracy of memory
A study carried out in the department of psychology at Yale University by Caset et al. (2018) aimed to investigate how working and long-term memory evolve across adolescence and the effect of different memory systems during this developmental period10. Researchers assessed participants aged 8 to 30 years old using tasks that measured working, immediate, and long-term memory. The task included recall of an item that was previously presented, and the participants had to either say if it was “new” (not presented before) or “old” (presented before). This activity calculated the participant’s ability to retain and recall information over varying time intervals. The findings demonstrated a significant improvement in working and recognition memory during adolescence, illustrating optimal cognitive development between the ages of 15 and 25.This indicates the teenage brain undergoes developmental changes that optimize memory functions, which are necessary for cognitive maturation.
The study provides strong evidence supporting the development of memory systems as it focuses on multiple memory systems and their functions. The broad age range (8-30) also allows researchers to compare memory performances throughout different age groups. However the lack of neurobiological correlation and account of neural mechanisms limits broader discussion and biological understanding. Factors such as socioeconomic status, stress exposure, or educational backgrounds were not reported, increasing sample variability, which makes it difficult for researchers to draw correlation between two factors.
Memory and neuroplastic connections are directly related; stronger neuroplastic connections in the hippocampal region of the brain enhance memory formation. Adolescents experience heightened dopamine release, which gives them more motivation to learn new skills like exploration, learning, and social interactions, which promote neuroplastic growth in the brain. The dopaminergic surge enhances the reward-motivation mechanism as it signals the brain to repeat certain rewarding behaviours. This is associated with a feeling of pleasure, which is strengthened in the presence of dopamine, leading to increased motivation. A study published by “Behavioural Pharmacy” examines neuron circuits involved in stress-induced PFC dopamine alterations and how they interact with dopamine levels, contributing to cognitive impairments like structural changes in the brain that affect memory11.
Biomarkers of stress
Factors like chronic stress significantly impact memory by impairing the ability to encode new information and retrieve older memories. Cortisol, which is a steroid hormone, plays a role in regulating blood pressure, glucose levels, and carbohydrate metabolism. When the brain is exposed to a stressor, it activates the hypothalamus, which releases corticotropin-releasing hormone (CRH). This hormone travels down to the kidneys and causes the adrenal glands to release ACTH, which stimulates the release of cortisol into the bloodstream. While the brain is experiencing stress for long durations (chronic stress), cortisol is exposed for very long times in the bloodstream, which can reduce hippocampal volume (decreased synaptic density) and make the retrieval and encoding of information challenging.
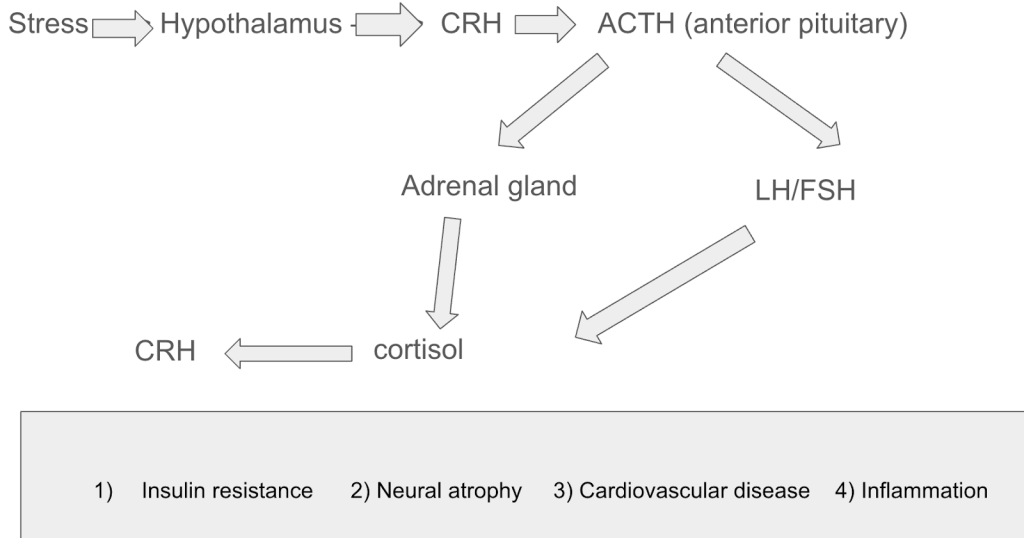
Research carried out by Cadu Klier and Luciano Grüdtner Buratto titled “Stress and Long-Term Memory Retrieval” aimed to investigate how stress-induced cortisol levels affected recall of long-term memories. The researchers reviewed 13 studies, including 962 healthy human participants, from PubMed and PsycNet13. They also performed a final memory test 15-20 minutes after the participants had been exposed to stressful conditions (acute psychosocial stress) during encoding of information. The evidence suggested that stress negatively impacts the retrieval of long-term memories, primarily due to increased cortisol levels.
The study uses a large sample size which enables researchers to increase generalizability and transferability of results. Secondly, the review of various other studies shows that researchers have considered multiple perspectives, which helps decrease bias and considers broadens perspective. However the short duration of exposure to a stressor limits the validity of this study as no follow up tests were conducted to assess any more structural or functional changes in the brain.
Epigenetic modifications in response to stress
Research suggests that epigenetic mechanisms play a crucial role in mediating the effects of chronic stress by altering gene expressions. These modifications can be influenced by various environmental factors and include changes caused due to mechanisms like, DNA methylation, histone modifications or non-coding RNA interference (Rettner, 2013). Stress – induced epigenetic changes, especially in the BDNF and CRHR1 genes have shown significant dysregulation of synaptic plasticity, memory deficits and cognitive development.
Effect of stress on BDNF gene expression
Chronic stress triggers the overactivation of the HPA axis, which leads to increased cortisol levels in the body. High cortisol exposure for longer periods of time initiates epigenetic mutations on the BDNF gene promoter region, via DNA methylation. In this process, a methyl group (-CH3) is added to the cytosine nucleotide of the gene. The overmethylation of the gene promoter region causes transcription factors, such as CREB, to bind efficiently, ultimately leading to downregulation and resulting in very low levels of the BDNF protein in the body, particularly in areas like the hippocampus, which are essential for memory.
Effect of epigenetic markers on the CRHR1 receptor promoter region
The corticotropin (CRH) type 1 receptor is known as the CRHR1, which plays a central role in coordinating endocrine, autonomic and behavioural responses to stress (Crhr1 Corticotropin Releasing Hormone Receptor 1 [Mus Musculus (House Mouse)] – Gene – NCBI). Changes in this epigenetic mark can cause increased stress or a greater possibility of being diagnosed with stress-related disorders.
DNA methylation occurs typically in the CpG sites (specific locations on the DNA which contain cytosine followed by guanine nucleotides in the 5’ to 3’ direction), which cause either hypermethylation – downregulation of the CRHR1 gene which dampens stress response or hypomethylation – upregulating the CRHR1 gene production, resulting in heightened stress response.
Stress can also induce changes in miRNAs (small RNA molecules responsible for regulating gene expression), which often impair or degrade the body’s ability to translate a specific protein. miR-34c is a type of miRNA which targets the untranslated cytosine in the 3′ untranslated region of the CRHR1 gene, decreasing its expression.
The role of environmental modulators in adolescent stress exposure
Although biological and developmental factors play a significant role in stress disorders, environmental modulators such as socioeconomic status (SES) also play a crucial role in shaping adolescents cognitive functions (Kaimo Ding a b et al., 2023). Adolescents belonging to a lower SES are prone to encounter more stressful environments such as competition for basic necessities (education, food, water, shelter, clothing), housing instability, violent neighbourhoods, academic pressure with limited educational resources and family instability. These persistent stressors have been exposed to the individual since childhood causing long-term alterations in the brain’s structure and function, due to overactivation of the HPA axis and chronic inflammation. Furthermore, adolescents belonging to lower SES usually do not have access to early intervention programs or supporting school based systems. The lack of access to mental healthcare accounts for a greater cumulative stress load increasing emotional deficits, decreasing memory retention and regulatory issues.
Hanson et al (2013) (Hanson JL; Hair N; Shen DG; Shi F; Gilmore JH; Wolfe BL; Pollak SD;) aimed to investigate how poverty and socioeconomic status affect early brain development (grey and white matter volumes) and social and cognitive outcomes in young children. The participants included 77 children from different economic backgrounds. This longitudinal study was carried out on children aged 5 months to 4 years. MRI scans for each child were taken 7 times, in order to measure total grey, white and cerebral volume. The data was analysed using a novel image processing algorithm. The results displayed that children from lower SES had reduced grey matter in the prefrontal cortex and parietal lobes, associating children from lower SES with slower brain growth over time.
Conclusion
Chronic stress exposure disrupts delicate neurochemical balances within the brain, particularly those involving cortisol and BDNF, which play central roles in neural plasticity and memory.An increase in cortisol levels disrupts the homeostasis of other functioning proteins like BDNF, a critical molecule for neuronal survival. The reduction of BDNF and excessive cortisol for a long period can cause the hippocampal volume and synaptic connections to decrease, which weakens memory formation and recall. This can negatively impact adolescents’ cognitive maturation, as the development of the brain does not occur properly.
Adolescents chronically exposed to stress often exhibit difficulties in learning, decision-making, and emotional regulation, alongside increased vulnerability to anxiety, depression, and poor academic outcomes. Encouraging physical activity like sports, dancing, or walking helps in increasing the regulation of endorphins, which improve mood and reduce stress. Maintaining adjacent sleep (at least 9 hours of continuous rest)14 allows the body to recharge and regulate stress hormones like cortisol, which improves emotional regulation and also strengthens emotional resilience as individuals are better equipped to handle daily stressors. Schools can also play a vital role in providing students with proper resources and therapists. This may include stress management workshops, support groups, or even a professional therapist or counselor present on the school campus. Friends and family can also play their role by creating a comfortable environment for students to express their feelings and feel safe to share their thoughts.
References
- The Editors of Encyclopaedia Britannica, 2025. [↩]
- Rai, D., Dey, S., & Ray, K. (2018). A method for estimating relative changes in the synaptic density in Drosophila central nervous system. BMC Neuroscience, 19(1). https://doi.org/10.1186/s12868-018-0430-3 [↩]
- Huttenlocher, Peter R. “Synaptic Density in Human Frontal Cortex – Developmental Changes.” ResearchGate, 1979 [↩]
- Huttenlocher, Peter R. “Synaptic Density in Human Frontal Cortex – Developmental Changes.” ResearchGate, 1979 [↩]
- Miranda, M., Morici, J. F., Zanoni, M. B., & Bekinschtein, P. (2019). Brain-Derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Frontiers in Cellular Neuroscience, 13. https://doi.org/10.3389/fncel.2019.00363 [↩]
- Marlatt, M. W., Potter, M. C., Lucassen, P. J., & Van Praag, H. (2012). Running throughout middle‐age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Developmental Neurobiology, 72(6), 943–952. https://doi.org/10.1002/dneu.22009 [↩] [↩]
- Miguez et al., 2020c [↩]
- MSEd, K. C. (2024b, June 19). What is memory? Verywell Mind. https://www.verywellmind.com/what-is-memory-2795006 [↩]
- Short-Term Memory Impairment. (2024, June). NCIB. Retrieved April 26, 2025, from https://www.ncbi.nlm.nih.gov/books/NBK545136/#:~:text=Introduction,includes%20semantic%20and%20episodic%20memory. [↩]
- Miguez et al., 2020d [↩]
- DIFFERENTIAL EFFECTS OF STRESS AND ANXIOGENIC DRUGS ON. . . : Behavioural Pharmacology, n.d. [↩]
- Hantsoo et al., “The Role of the Hypothalamic-pituitary-adrenal Axis in Depression Across the Female Reproductive Lifecycle: Current Knowledge and Future Directions” [↩]
- Klier, Cadu, and Luciano Grüdtner Buratto. “Stress and Long-term Memory Retrieval: A Systematic Review.” Trends in Psychiatry and Psychotherapy, vol. 42, no. 3, Sept. 2020, pp. 284–91. https://doi.org/10.1590/2237-6089-2019-0077. [↩]
- Rd, T. J. (2024, August 6). How many hours of sleep do you really need? Healthline. https://www.healthline.com/nutrition/how-much-sleep-you-need [↩]