Abstract
Immunocompromised individuals face significant challenges in mounting effective immune responses to traditional vaccines, leaving them vulnerable to severe infections. This limitation prompts the need for alternative immunization strategies. While passive immunization using therapeutic monoclonal antibodies (mAbs) offers immediate protection, its clinical utilization faces challenges related to production complexity and cost. Messenger RNA (mRNA)-based antibody therapeutics represent a novel approach to passive immunization, offering rapid and targeted protection without relying on the recipient’s immune system. This review outlines the challenges for immunizing immunocompromised populations and explores the mechanisms, current developments, advantages, challenges, and future directions of mRNA-based antibody therapeutics for passive immunization.
Keywords: mRNA-based antibodies, passive immunization, immunocompromised populations,
Introduction
Immunity is the body’s natural defense mechanism against infections and disease. Two primary approaches for inducing immunity are active immunization and passive immunization. Active immunization works by stimulating the body’s own immune system to produce antibodies, typically through vaccination, leading to a slow onset of protection over days to weeks. But it offers long-lasting immunity and forms memory cells for future defense. In contrast, passive immunization involves the direct administration of antibodies, providing immediate protection within hours but with limited duration from weeks to months, and it does not generate immunological memory.
Immunocompromised individuals, including those with human immunodeficiency virus (HIV) infection, cancer, organ transplants, primary immunodeficiencies, and patients treated with immunosuppressive biologics or medications, are at increased risk for severe infections due to impaired or weakened immune responses1. While vaccination is the most effective tool for preventing infectious diseases in the general population, traditional vaccines often fail to elicit protective immune responses in these populations due to impaired B- and T-cell function and, in some cases, may pose safety risks2. The specific immunological challenges of these subgroups are outlined below:
HIV-Positive Patients: HIV leads to the depletion or dysfunction of CD4+ T helper cells, impairing both antibody production and cellular immunity. While inactivated vaccines are typically recommended, individuals, especially those with advanced diseases, exhibit lower seroconversion rates and reduced efficacy. Live attenuated vaccines are generally avoided due to the risk of uncontrolled replication of the vaccine strain3.
Cancer Patients: Immune suppression in cancer patients varies by tumor type and treatment. Hematological cancers such as leukemia and lymphoma directly impair immune function, while chemotherapy and radiation therapy cause broad, temporary immune suppression. As a result, vaccine efficacy is often inconsistent and reduced in this population4.
Organ Transplant Recipients: These individuals require lifelong immunosuppressive therapy to prevent graft rejection, broadly suppressing both B- and T-cell activity. Vaccine responses are markedly reduced, and live attenuated vaccines are contraindicated due to the risk of systemic infection in immunosuppressed hosts5.
Patients with Primary Immunodeficiencies: These inherited disorders result in defective immune components and highly variable vaccine responses. For instance, patients with antibody deficiencies show impaired humoral responses, while those with combined immunodeficiencies such as severe combined immunodeficiency (SCID) exhibit profound deficits in both B- and T- cells, rendering most vaccines ineffective. Live attenuated vaccines are generally avoided in this group due to safety concerns6.
Patients Treated with Immunosuppressive Biologics and Medications: Targeted immunosuppressive therapies used to treat autoimmune and inflammatory conditions can diminish vaccine-induced immunity. The extent of impairment depends on the mechanism of action, dosage, and duration of treatment. For example, B-cell depleting agents substantially reduce antibody production, while TNF-α and interleukin inhibitors can blunt both humoral and cellular responses7.
In addition to passive immunization using monoclonal antibodies (mAbs), advances in mRNA-based antibody therapeutics offer a promising alternative. It uses mRNA to instruct cells to produce therapeutic proteins including antibodies that can treat or prevent diseases without relying on the recipient’s immune system. mRNA technology has attracted significant attention due to its success in developing COVID-19 vaccines and its potential in other therapeutic areas, such as cancer, genetic disorders, and infectious diseases. Utilizing this platform to encode therapeutic antibodies presents a promising strategy for achieving rapid, scalable, and potentially more cost-effective passive immunity compared to traditional mAbs8,9,10,11.
This review provides a comprehensive overview of the development of mRNA-based antibody therapeutics for passive immunization, with particular emphasis on their potential application in immunocompromised populations. It also explores the key advantages, challenges, and future directions for clinical implementation.
Methods
A comprehensive literature search was conducted using PubMed, Web of Science, and Google Scholar with keywords “mRNA”, “antibody therapeutics”, and “passive immunization” or “passive vaccination”. The search was initiated in January 2025 and covered studies published from 2017 to 2025. Papers were screened based on their title and abstracts for relevant information focusing on mRNA-based antibody therapeutics, advantages, and challenges. Both original research articles and reviews that provided substantial insights into the topics were selected during the screening stage. Specific criteria for inclusion were studies on mRNA-based antibodies for passive immunization with animal or human data. Studies on mRNA vaccines for active immunization or mRNA therapeutics for cancer and genetic disorders are excluded.
Passive immunization using mRNA-based Antibodies
Passive Immunization using Antibodies
Passive immunization using mAbs presents a critical therapeutic strategy for immunocompromised patients who are unable to generate robust immune responses. However, its broader application is limited by significant challenges such as high product cost, complex manufacturing, and stability issues12.
To address these limitations, several next-generation antibody technologies have emerged as alternative strategies for passive immunization. Recombinant mAbs are produced using genetic engineering techniques, enabling enhanced batch-to-batch consistency and greater scalability. Fc-engineered IgGs incorporate modifications in the Fc (constant) region to prolong antibody half-life or improve effector functions, such as antibody-dependent cellular cytotoxicity. Bispecific antibodies (notably bispecific T-cell engagers) are designed to simultaneously bind two different targets, typically one on a pathogen or diseased cell and the other on an immune effector cell, thereby promoting targeted cytotoxicity without relying solely on viral neutralization13. Viral vector-mediated antibody delivery, particularly using adeno-associated virus (AAV), enables sustained in vivo antibody expression. However, this approach is limited by the development of immune responses to both the antibody and the viral vector, which can reduce efficacy and prevent redosing. There are also theoretical concerns regarding genomic integration near oncogenes, along with challenges related to complex manufacturing requirements. Plasmid DNA-based antibody delivery involves introducing plasmids encoding antibody genes into host cells. These are transcribed and translated into functional antibodies using a non-viral platform. This method offers several advantages, including extended expression from weeks to months, low production cost, room-temperature stability, and repeat dosing with minimal immunogenicity. However, it faces limitations such as low transfection efficiency, potential risks of genomic integration, and a delayed onset of action as the process requires nuclear entry and transcription, which can take several days to weeks. These limitations make it less ideal for use in urgent or acute settings14.
mRNA-based Antibody Therapeutics
mRNA-based antibody represents a revolutionary approach for passive immunization that harnesses mRNA technology to produce antibodies directly within the body. It combines the advantages of mRNA technology with the immediate protective effects of antibodies.
Rather than injecting the antibody protein itself, mRNA encoding the heavy and light chains of the specific antibody is delivered to instruct host cells to produce a full-length monoclonal antibody or antibody fragment. Due to its negative charge and high molecular weight, mRNA cannot cross the cellular membranes to reach the cytosol, necessitating the use of a delivery vector. Although various delivery vectors have been developed in preclinical studies, lipid nanoparticles (LNPs) remain the preferred delivery system for clinical use. LNPs effectively protect mRNA from RNase degradation and facilitate its cellular delivery and endosomal escape15. Once administered (usually intravenously, intramuscularly, or via inhaled route), the LNPs are endocytosed by host cells, releasing the mRNA into the cytoplasm, where it is translated into functional antibodies. These antibodies are then secreted into the blood circulation or mucosal surfaces, providing immediate protection (Figure 1). In vivo antibody expression from mRNA can be detected as early as 2 hours post-administration, peak expression around 24 to 48 hours, and last for several days16.
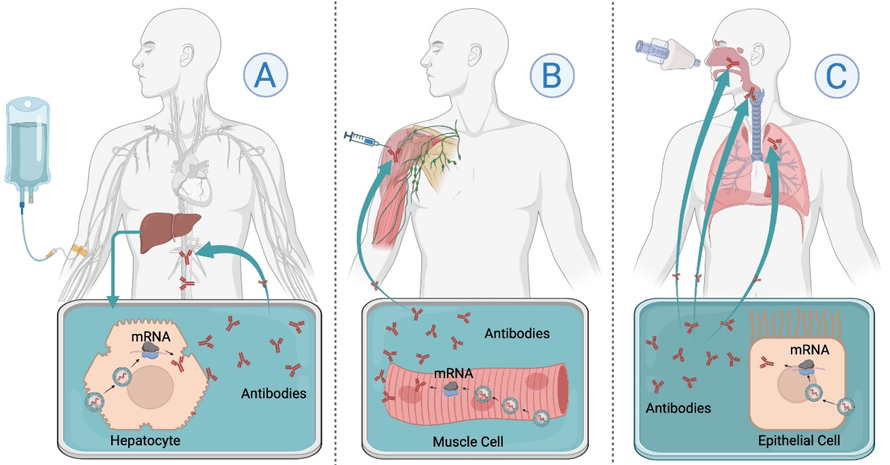
A: Intravenous (IV) administration delivers mRNA-LNPs to the liver, where hepatocytes translate the mRNA into antibodies that enter blood circulation; B: Intramuscular (IM) injection introduces mRNA into muscle cells, leading to localized antibody production at the injection site with some blood distribution. C: Aerosolized or intranasal delivery targets epithelial cells in the respiratory tract, enabling localized antibody production at mucosal surfaces to enhance lung immunity.
Pre-clinical and Clinical Research
mRNA vaccines have been approved and widely used in the human population and have played an important role in preventing COVID-19 spread worldwide. Unlike mRNA vaccines that deliver antigens to trigger active immunity, mRNA-based antibody therapeutics are used to elicit passive immunity by directing host cells to produce protective antibodies. The Weissman group was the first to publish the feasibility of in vivo delivery of antibodies using the mRNA-LNP platform in 2017. The data showed that intravenous injection of LNP-formulated N1-methylpseudouridine (m1Ψ)-modified mRNA encoding the heavy and light chains of the broadly neutralizing anti-HIV antibody VRC01 led to detectable expression within hours, peaking at 24 hours, and weekly dosing sustained therapeutic antibody levels in BALB/c mice. A single injection protected humanized mice from intravenous HIV-1 challenge8. These findings highlight the potential of mRNA-based antibodies to rapidly induce protective immunity, supporting both prophylactic and therapeutic applications. Since then, there have been several pre-clinical studies using mRNA-based antibodies for passive immunization against viruses including rabies9, respiratory syncytial virus (RSV)17, influenza10, HIV18, Zika19, SARS-CoV-220,21,22 and Hepatitis B (HBV)23, and bacteria such as salmonella enterica serovar typhimurium (STm)24 and pseudomonas aeruginosa24,25.
Most mRNA-based antibody therapies have used intravenous (IV) administration, targeting the liver as the main site of expression. However, for respiratory infections like SARS-CoV-2, RSV, and influenza, lung-targeted delivery is especially important. Several approaches have been developed to localize antibody expression in the lungs, including nebulizer delivery26, aerosolized mRNA17, intratracheal (IT)10, and intranasal (IN) administration20. Vanover et al. developed an inhalable formulation to enable lung-localized expression of mRNA-encoded, membrane-anchored neutralizing antibodies (COV2-2832 or DH1041) for SARS-CoV-2. In hamsters, prophylactic nebulized delivery significantly reduced weight loss, viral titers, and lung pathology. Although small animal models present challenges in aerosol distribution, mostly limited to the alveolar space, this limitation is expected to be reduced in larger species, which allow for broader respiratory tract targeting26. Another RSV study used aerosolized naked mRNA to deliver neutralizing antibodies directly to the lung. This modular, synthetic approach led to antibody expression within 24 hours and persistence for up to 28 days in mice. All tested constructs significantly reduced viral load, with some achieving complete clearance. Anchored single-chain antibodies (RSV aVHH) exhibited enhanced lung retention and durable protection. These results support aerosol mRNA delivery as a promising method for lung-targeted prophylaxis10.
While intramuscular delivery could offer practical advantages for rapid deployment in outbreak settings, it has shown low efficacy for mRNA-based antibodies due to the limited number of target cells at the injection site. To address this, Erasmus et al. used a self-amplifying alphavirus-derived replicon RNA (repRNA) to boost per-cell expression of the ZIKV-117 antibody. This approach achieved serum antibody levels more than 30 times higher than those produced with conventional mRNAs and protected mice from lethal Zika virus challenge. Although self-amplifying mRNA may also offer longer expression, this study did not investigate time points beyond 10 days19. If successfully translated to larger models and humans, self-amplifying mRNA could enable scalable, rapid passive immunization during epidemics.
Most mRNA-based antibody research has focused on delivering IgG, which provides robust systemic protection against bloodstream and respiratory pathogens. However, IgA is the dominant antibody at mucosal surfaces and may offer improved localized immunity. Deal et al. developed an mRNA-based platform encoding IgA heavy, light, and joining (J) chains within LNPs to produce pathogen-specific IgA antibodies in mucosal secretions. In animal models, Salmonella-specific IgA limited intestinal invasion, while Pseudomonas-targeted IgA protected the lung infection27. While these findings are promising, the study used high doses in mice (1 mg/kg), exceeding those tested in humans (0.1–0.6 mg/kg)28, which raise potential safety concerns that require further investigation. Additionally, the platform was evaluated only in prophylactic models, leaving its therapeutic potential untested.
Across preclinical studies, mRNA-based antibodies were typically administered as a single dose via intravenous, intramuscular, aerosol, or intranasal routes, with doses ranging from 10 μg to 1 mg/kg depending on the delivery method and animal model. Antibody expression generally began within 2 to 24 hours and lasted from several days to over 60 days, with some formulations showing protection for up to 16 weeks. Most studies reported strong protective effects, including reduced viral titers, improved survival, and prevention of infection. Adverse effects were minimal, with only mild and transient cytokine responses observed in a few cases, resolving without tissue damage or systemic inflammation. Importantly, anti-drug antibody (ADA) responses were rare, and even when present (as in a rabies model), no serious adverse events occurred, underscoring the platform’s safety and tolerability. However, human translation of mRNA-based antibodies for passive immunization faces challenges including immune responses (e.g., ADAs), variability in expression, and pre-existing immunity to delivery components. Further work is needed to optimize dosing, ensure long-term safety, and achieve consistent expression across diverse patient populations.
Moderna’s mRNA-1944, an mRNA-LNP encoding the CHKV-24 neutralizing antibody against chikungunya virus, became the first therapeutic mRNA antibody to enter human clinical trials. The Phase 1, randomized, placebo-controlled, dose-escalation study in healthy adults showed sustained antibody expression, functional neutralizing activity, and good safety, supporting its potential as a long-acting treatment for chikungunya virus. It may offer passive immunization for chikungunya virus. Future studies are required to assess its protective efficacy in larger populations, including those in endemic regions28.
These preclinical and clinical studies (Table 1) indicate that mRNA-based passive immunization could be potentially used for immunocompromised populations that cannot benefit from active immunization.
| Infectious Disease | Antibody | Model | Key Outcome | Ref (Year) |
| HIV | IgG (VRC01) | Humanized mice with HIV-1 challenge | A single IV dose of 30 μg mRNA-LNP; Peak NA levels at 24 h, lasting up to 7 days; Fully protected from HIV-1 infection; No systemic immune activation observed; No ADAs detected after repeated dosing. | (2017)29 |
| HIV | IgG (aPGT121) | Simian-HIV models in macaques | A single aerosol delivery of 1,000 μg naked mRNA; Neutralization by 4 hours; lasting up to 28 days; Mucosal protection for prevention of HIV infections. | (2020)30 |
| Rabies | IgG (S057) | Mouse model with rabies challenge | A single IV dose of 40 μg mRNA-LNP; NA levels within 2 hours, peaking at 6–12 hours and lasting up to 4 weeks in about half the mice; Full protection in prophylactic and early post-exposure models. Transient, mild cytokine elevations; In some mice, ADA responses led to faster antibody clearance, but no overt adverse events were reported. | (2017)31 |
| RSV | IgG (RSV-F); RSV aVHH | RSV infection mice model | A single aerosol delivery of 100 μg naked mRNA; Ab began within 24 hours, lasting up to 28 days; Protect mice challenged 7 days post-transfection. RSV aVHH reduced viral titers and lung severity; No significant cytokine induction or histological abnormalities; No immune toxicity or inflammatory responses. | (2018)32 |
| Influenza | Bispecific VHH antibody | Mice with influenza challenge | A single IT dose of 5 μg mRNA-LNP; Ab peaked at 6 hours and lasting for up to 48 hours; Reduced weight loss, mortality, and lung viral titers; Protection up to 48 hours post-infection; A transient IL-6 spike and temporary increase in granulocytes resolved within 24 hours. | (2020)33 |
| Zika | IgG (ZIKV-117) | Mice infected with ZIKV | A single IM dose of 4 μg repRNA-LNPs; Protection prophylactically or 1 day after infection; Fully preventing viremia and death in mice. | (2020)34 |
| Chikungunya | IgG (CHKV-24, mRNA-1944) | Healthy adults (N =38; Age 18-50 years) | A single IV dose of LNP-mRNA at 0.1–0.6 mg/kg; Dose-dependent expression of neutralizing IgG; Reached therapeutic levels within 12–48 hours and persisted for at least 16 weeks at higher doses. | (2021)35 |
| SARS-CoV-2 | IgG (CB6) | Mice infected with SARS-CoV-2 | A single IN dose 5 × 10⁵ IU of VEEV-VRP; Local lung expression of the NA within 24 hours, lasting at least 5 days; Reduced viral titers, no pathology in treated mice. | (2021)36 |
| SARS-CoV-2 | IgG (HB27) | Mice and hamsters infected with SARS-CoV-2 | A single IV dose of mRNA-LNP at 0.2 or 1 mg/kg; NA peaked at day 7, t1/2 of ~15 days, up to 63 days; Full protection in mice; in hamsters, 0.3–1 mg/kg dose prevents virus transmission and lung pathology. | (2022)37 |
| SARS-CoV-2 | IgG (COV2-2832 or DH1041) | Hamsters infected with SARS-CoV-2 | A single 312 µg dose of nebulizer polymer-mRNA; Significant reduction in viral RNA and lung pathology when challenged 2 days post. | (2022)38 |
| HBV | G12-scFv; G12-scFv-Fc; G12-IgG | Mice with HBV infection | A single IV dose of mRNA-LNPs at 2.5 mg/kg; Ab expression for over 8 hours, t1/2 up to 58 hours; Reductions in HBsAg and HBV DNA levels lasting up to 30 days; G12-IgG and G12-scFv-Fc in LNPs were the most effective. | (2022)39 |
| ST; P. aeruginosa | IgA | Mouse Salmonella and pneumonia models | A single IV dose of 1 mg/kg mRNA-LNP; IgA2mRNA reduced ST intestinal invasion; IgA1mRNA and IgG1mRNA conferred mucosal protection in a P. aeruginosa pneumonia model. | (2023)40 |
| P. aeruginosa | scFV (anti-PcrV) | Mouse P. aeruginosa infection model | A single IV dose of 10 µg scFv-m166-LNPs; Prophylactic administration conferred 100% survival at 24 hours, while therapeutic use (30 minutes post-infection) achieved up to 80% survival over 7 days; Significantly reduced lung inflammation, bacterial burden, and cytokine levels (IL-6, TNF-α); Remained effective in immunocompromised mice. | (2025)41 |
Abbreviations: Ab, antibody; ADAs, anti-drug antibodies; aPGT, membrane anchored PGT; aVHH, an anchored, single-variable, heavy chain-only; Fc, fragment crystallizable; HBsAg, Hepatitis B surface antigen; IL-6, Interleukin-6; IM, intramuscular; IN, Intranasal; IT, intratracheal; IU, International Units; IV, intravenous; LNP, lipid nanoparticle; NA, Neutralizing Antibody; repRNA, Replicon RNA; RSV-F, respiratory syncytial virus fusion protein; scFv, single-chain variable fragment; ST, Salmonella Typhimurium; VEEV, Venezuelan equine encephalitis virus; VRP, alphavirus replicon particle; ZIKV, zika virus.
Advantages of mRNA-based Antibody Therapeutics
mRNA-based antibody therapeutics offer several compelling advantages over traditional monoclonal antibody production. The manufacturing process is highly efficient and flexible. mRNA constructs can be easily designed and modified by generating appropriate genomic sequences, and they can be rapidly synthesized using in vitro transcription (IVT) technology. Clinical batches can be produced within weeks after obtaining the sequence encoding the antibodies of interest, significantly accelerating development timelines. In addition, it enables in vivo production of antibodies, offering advantages such as reducing cost, a cell-free process, and eliminating the need for the traditional production, scalability, and purification processes currently used for antibodies13. It is amenable to customizable designs, and it can be used to deliver mRNA-encoded specific antibodies, antibody fragments, or bispecific antibodies with broader therapeutic effects11. Furthermore, it is a platform technology that allows for quick updates by simply changing the mRNA sequence without major re-engineering of the manufacturing process. Therefore, it is possible to expand the scope of passive immunization to other viruses or viral variants42.
Compared to DNA-based antibody delivery, which requires nuclear entry to be effective and carries a risk of integration, mRNA is taken up directly into the cytosol, enabling rapid protein production without entering the nucleus or posing a risk of genomic integration. This makes it a safer and more controllable platform43. Once delivered, it enables host cells to produce large quantities of properly folded and post-translationally modified antibodies, with expression durations that can be controlled from days to weeks.
Unlike vaccines that require weeks to develop immunity, mRNA-encoded antibodies can provide rapid protection with no requirement for host immune activation, which makes them a promising option for immunocompromised individuals whose immune systems are not effective in responding to vaccination.
Challenges, Limitations and Perspectives of mRNA-based Antibody Therapeutics
Despite the significant promise of mRNA-based antibody therapeutics, several critical limitations must be addressed before widespread clinical adoption is feasible, particularly in immunocompromised populations.
One of the main challenges is the efficient delivery of mRNA to target cells. While LNPs have emerged as the primary delivery system for mRNA vaccines and therapeutics, but the administration of LNPs containing mRNA-encoded antibodies has been mostly limited to liver-targeting via the intravenous route with infusions lasting an hour or more, which limits its scalability and access. Expanding delivery beyond the liver is essential to enable mRNA-based antibody therapeutics for broader infectious disease targets and vulnerable patient groups.
In addition, expression from mRNA-based therapeutics is transient, typically lasting from several days to a few weeks. Unlike vaccines, which stimulate the immune system to produce long-lived memory responses, passive immunization with mRNA antibodies offers no adaptive memory. This limits long-term protection and necessitates repeat dosing, which introduces additional complexity for long-term care, especially in high-risk patients who may require sustained prophylaxis but are already burdened by frequent clinical interventions43.
Innate immune activation poses another key challenge. Although nucleoside modifications (e.g., pseudouridine) reduce innate immune recognition, mRNA-based antibody therapeutics can still activate pathways like toll-like receptor 7 (TLR-7) or retinoic acid-inducible gene 1 (RIG-I), especially in immunocompromised individuals. Activation of the innate immune pathways could interfere with therapeutic applications by decreasing antibody expression and undermining tolerability. In certain cases, such as organ transplants, recipients may face increased risk of graft rejection due to inflammation or complement activation triggered by repeated LNP dosing16.
Beyond delivery and immunological concerns, there are also significant manufacturing and deployment challenges. While the rapid synthesis of mRNA offers speed and flexibility, cold-chain logistics, batch-to-batch consistency, and cost per dose remain substantial obstacles, especially for global implementation.
Nevertheless, the rapid progress made during the development of mRNA COVID-19 vaccines has catalyzed advances in mRNA synthesis, purification, and delivery technologies. These innovations can be leveraged to overcome current limitations and accelerate the development of mRNA-based antibody therapeutics. By addressing these challenges, especially those related to delivery, durability, immune tolerance, stability, and cost, this platform holds significant potential to provide scalable, rapid, and effective immune protection for immunocompromised individuals who are underserved by traditional vaccines.
Conclusion
Passive immunization using mRNA-based antibodies represents a transformative strategy for protecting immunocompromised individuals, particularly those who cannot mount sufficient responses to conventional vaccines. Preclinical studies in mice and hamsters, along with early-phase human trials, have demonstrated the feasibility, safety, and efficacy of mRNA-encoded antibody expression. However, realizing the full clinical potential of this approach requires overcoming several barriers. Key priorities include developing organ-specific LNP formulations to enhance tissue targeting (e.g., lung or mucosal delivery), designing longer-lasting mRNA constructs or utilizing self-amplifying mRNA to extend antibody expression, and optimizing delivery vehicles to minimize innate immune activation. Future research should prioritize clinical trials in high-risk populations, such as immunocompromised patients undergoing chemotherapy or transplant recipients, where conventional vaccine efficacy is limited. With continued investment and cross-disciplinary innovation, mRNA-based antibody therapeutics could soon become an important tool for rapid and scalable immune protection, particularly for vulnerable populations in the face of emerging infectious diseases.
References
- C. Agrati, B. Bartolini, V. Bordoni, F. Locatelli, M. R. Capobianchi, A. Di Caro, C. Castilletti, G. Ippolito. Emerging viral infections in immunocompromised patients: A great challenge to better define the role of immune response, Frontiers in Immunology.14, 1147871 (2023). [↩]
- S. Shoham, C. Batista, Y. Ben Amor, Ö. Ergönül, M. Hassanain, P. Hotez, G. Kang, J. H. Kim, B. Lall, H. J. Larson, D. Naniche, T. Sheahan, N. Strub-Wourgaft, S. O. Sow, A. Wilder-Smith, P. Yadav, M. E. Bottazzi. Vaccines and therapeutics for immunocompromised patients with COVID-19. eClinicalMedicine. 59, 101965 (2023). [↩]
- Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents With HIV [↩]
- L. Sarkar, V. B. Goli, N. Menon, V. M. Patil, V. Noronha, K. Prabhash. Vaccination practices, efficacy, and safety in adults with cancer: A narrative review. Cancer Research, Statistics, and Treatment. 4(3), 505–515 (2021). [↩]
- T. Velikova, S. Gerasoudis, H. Batselova. Vaccination for solid organ transplanted patients: Recommendations, efficacy, and safety. World Journal of Transplantation. 14(4), 92172 (2024). [↩]
- A. Sobh, F. A. Bonilla. Vaccination in primary immunodeficiency disorders. J Allergy Clinic Immunology In Practice. 4(6), 1066–1075 (2016). [↩]
- G. Blanchard‑Rohner. Vaccination in children with autoimmune disorders and treated with various immunosuppressive regimens: A comprehensive review and practical guide. Frontiers Immunology. 12, 711637 (2021). [↩]
- N. Pardi, A. J. Secreto, X. Shan, F. Debonera, J. Glover, Y. Yi, H. Muramatsu, H. Ni, B. L. Mui, Y. K. Tam, F. Shaheen, R. G. Collman, K. Karikó, G. A. Danet-Desnoyers, T. D. Madden, M. J. Hope, D. Weissman. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nature Communications. 8, 14630 (2017). [↩] [↩]
- M. Thran, J. Mukherjee, M. Pönisch, K. Fiedler, A. Thess, B. L. Mui, M. J. Hope, Y. K. Tam, N. Horscroft, R. Heidenreich, M. Fotin-Mleczek, C. B. Shoemaker, T. Schlake. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Molecular Medicine. 9, 1434–1447 (2017). [↩] [↩]
- L. Van Hoecke, R. Verbeke, D. De Vlieger, H. Dewitte, K. Roose, S. Van Nevel, O. Krysko, C. Bachert, B. Schepens, I. Lentacker, X. Saelens. mRNA encoding a bispecific single domain antibody construct protects against influenza A virus infection in mice. Molecular Therapy Nucleic Acids. 20, 777–787 (2020). [↩] [↩] [↩] [↩]
- Y. Zhao, L. Gan, D. Ke, Q. Chen, Y. Fu. Mechanisms and research advances in mRNA antibody drug-mediated passive immunotherapy. Journal of Translational Medicine. 21, 693 (2023). [↩] [↩]
- V. Sifniotis, E. Cruz, B. Eroglu, V. Kayser. Current advancements in addressing key challenges of therapeutic antibody design, manufacture, and formulation. MDPI, Antibodies. 8(2), 36 (2019). [↩]
- R. Singh, P. Chandley, S. Rohatgi. Recent advances in the development of monoclonal antibodies and next‑generation antibodies. ImmunoHorizons. 7(12), 886–897 (2023). [↩] [↩]
- C. Chung, S. B. Kudchodkar, C. N. Chung, Y. K. Park, Z. Xu, N. Pardi, M. Abdel-Mohsen, K. Muthumani. Expanding the reach of monoclonal antibodies: A review of synthetic nucleic acid delivery in immunotherapy. MDPI, Antibodies. 12(3), 46 (2023). [↩]
- Y. Shi, M. Shi, Y. Wang, J. You. Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications. Signal Transduction and Targeted Therapy. 9, 322 (2024). [↩]
- C. E. Deal, A. Carfi, O. J. Plante. Advancements in mRNA encoded antibodies for passive immunotherapy. MDPI, Vaccines. 9(2), 108 (2021). [↩] [↩]
- P. M. Tiwari, D. Vanover, K. E. Lindsay, S. S. Bawage, J. L. Kirschman, S. Bhosle, A. W. Lifland, C. Zurla, P. J. Santangelo. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nature Communications. 9, 3999 (2018). [↩] [↩]
- K. E. Lindsay, D. Vanover, M. Thoresen, H. King, P. Xiao, P. Badial, M. Araínga, S. B. Park, P. M. Tiwari, H. E. Peck, E. L. Blanchard, J. M. Feugang, A. K. Olivier, C. Zurla, F. Villinger, A. R. Woolums, P. J. Santangelo. Aerosol delivery of synthetic mRNA to vaginal mucosa leads to durable expression of broadly neutralizing antibodies against HIV. Molecular Therapy. 28(3), 805–819 (2020). [↩]
- J. H. Erasmus, J. Archer, J. Fuerte-Stone, A. P. Khandhar, E. Voigt, B. Granger, R. G. Bombardi, J. Govero, Q. Tan, L. A. Durnell, R. N. Coler, M. S. Diamond, J. E. Crowe, S. G. Reed, L. B. Thackray, R. H. Carnahan, N. Van Hoeven. Intramuscular delivery of replicon RNA encoding ZIKV-117 human monoclonal antibody protects against Zika virus infection. Molecular Therapy Methods and Clinical Development. 18, 402–414 (2020). [↩] [↩]
- J.-Q. Li, Z.-R. Zhang, H.-Q. Zhang, Y.-N. Zhang, X.-Y. Zeng, Q.-Y. Zhang, C.-L. Deng, X.-D. Li, B. Zhang, H.-Q. Ye. Intranasal delivery of replicating mRNA encoding neutralizing antibody against SARS-CoV-2 infection in mice. Signal Transduction and Targeted Therapy. 6, 369 (2021). [↩] [↩]
- Y.-Q. Deng, N. Zhang, Y. Zhang, Y.-X. Ye, X. Zhang, X. Xue, H. Xu, D.-D. Liu, X.-J. Zhao, Y. Cao, H. Chen, C. Qin, B. Ying. Lipid nanoparticle-encapsulated mRNA antibody provides long-term protection against SARS-CoV-2 in mice and hamsters. Nature, Cell Research. 32, 375–382 (2022). [↩]
- D. Vanover, C. Zurla, H. E. Peck, N. Orr‑Burks, J. Y. Joo, J. Murray, N. Holladay, R. A. Hobbs, Y. Jung, L. C. S. Chaves, L. Rotolo, A. W. Lifland, A. K. Olivier, D. Li, K. O. Saunders, G. D. Sempowski, J. E. Crowe Jr., B. F. Haynes, E. R. Lafontaine, R. J. Hogan, P. J. Santangelo. Nebulized mRNA‑encoded antibodies protect hamsters from SARS‑CoV‑2 infection. Advanced Science. 9, e2202771 (2022). [↩]
- B. Chen, Y. Chen, J. Li, C. Wang, W. Song, Y. Wen, J. Lin, Y. Wu, T. Ying. A single dose of anti-HBsAg antibody-encoding mRNA-LNPs suppressed HBsAg expression: a potential cure of chronic hepatitis B virus infection. mBio. 13(4), e01612-22 (2022). [↩]
- C. E. Deal, A. F. Richards, T. Yeung, M. J. Maron, Z. Wang, Y.-T. Lai, B. R. Fritz, S. Himansu, E. Narayanan, D. Liu, R. Koleva, S. Licht, C. J. Hsiao, I. L. Rajlic, H. Koch, M. Kleyman, M. E. Pulse, W. J. Weiss, J. E. Doering, S. K. Lindberg, N. J. Mantis, A. Carfi, O. J. Plante. An mRNA‑based platform for the delivery of pathogen‑specific IgA into mucosal secretions. Cell Reports Medicine. 4(11), 101253 (2023). [↩] [↩]
- M. Kinoshita, K. Kawaguchi, B. T. Nguyen Le, A. Kainuma, T. Sawa, S. Uchida. Fc-free single chain antibody mRNA therapy for airway infection of multidrug-resistant Pseudomonas aeruginosa. bioRxiv. 659416 (2025). [↩]
- D. Vanover, C. Zurla, H. E. Peck, N. Orr‑Burks, J. Y. Joo, J. Murray, N. Holladay, R. A. Hobbs, Y. Jung, L. C. S. Chaves, L. Rotolo, A. W. Lifland, A. K. Olivier, D. Li, K. O. Saunders, G. D. Sempowski, J. E. Crowe Jr., B. F. Haynes, E. R. Lafontaine, R. J. Hogan, P. J. Santangelo. Nebulized mRNA‑encoded antibodies protect hamsters from SARS‑CoV‑2 infection. Advanced Science. 9, e2202771 (2022). [↩] [↩]
- C. E. Deal, A. F. Richards, T. Yeung, M. J. Maron, Z. Wang, Y.-T. Lai, B. R. Fritz, S. Himansu, E. Narayanan, D. Liu, R. Koleva, S. Licht, C. J. Hsiao, I. L. Rajlic, H. Koch, M. Kleyman, M. E. Pulse, W. J. Weiss, J. E. Doering, S. K. Lindberg, N. J. Mantis, A. Carfi, O. J. Plante. An mRNA‑based platform for the delivery of pathogen‑specific IgA into mucosal secretions. Cell Reports Medicine. 4(11), 101253 (2023). [↩]
- A. August, H. Z. Attarwala, S. Himansu, S. Kalidindi, S. Lu, R. Pajon, S. Han, J.-M. Lecerf, J. E. Tomassini, M. Hard, L. M. Ptaszek, J. E. Crowe, T. Zaks. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nature Medicine. 27, 2224–2233 (2021). [↩] [↩]
- N. Pardi, A. J. Secreto, X. Shan, F. Debonera, J. Glover, Y. Yi, H. Muramatsu, H. Ni, B. L. Mui, Y. K. Tam, F. Shaheen, R. G. Collman, K. Karikó, G. A. Danet-Desnoyers, T. D. Madden, M. J. Hope, D. Weissman. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nature Communications. 8, 14630 (2017). https://doi.org/10.1038/ncomms14630 [↩]
- K. E. Lindsay, D. Vanover, M. Thoresen, H. King, P. Xiao, P. Badial, M. Araínga, S. B. Park, P. M. Tiwari, H. E. Peck, E. L. Blanchard, J. M. Feugang, A. K. Olivier, C. Zurla, F. Villinger, A. R. Woolums, P. J. Santangelo. Aerosol delivery of synthetic mRNA to vaginal mucosa leads to durable expression of broadly neutralizing antibodies against HIV. Molecular Therapy. 28(3), 805–819 (2020). https://doi.org/10.1016/j.ymthe.2020.01.002 [↩]
- M. Thran, J. Mukherjee, M. Pönisch, K. Fiedler, A. Thess, B. L. Mui, M. J. Hope, Y. K. Tam, N. Horscroft, R. Heidenreich, M. Fotin-Mleczek, C. B. Shoemaker, T. Schlake. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Molecular Medicine. 9, 1434–1447 (2017). https://doi.org/10.15252/emmm.201707678 [↩]
- P. M. Tiwari, D. Vanover, K. E. Lindsay, S. S. Bawage, J. L. Kirschman, S. Bhosle, A. W. Lifland, C. Zurla, P. J. Santangelo. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nature Communications. 9, 3999 (2018). https://doi.org/10.1038/s41467-018-06508-3 [↩]
- L. Van Hoecke, R. Verbeke, D. De Vlieger, H. Dewitte, K. Roose, S. Van Nevel, O. Krysko, C. Bachert, B. Schepens, I. Lentacker, X. Saelens. mRNA encoding a bispecific single domain antibody construct protects against influenza A virus infection in mice. Molecular Therapy Nucleic Acids. 20, 777–787 (2020). https://doi.org/10.1016/j.omtn.2020.04.015 [↩]
- J. H. Erasmus, J. Archer, J. Fuerte-Stone, A. P. Khandhar, E. Voigt, B. Granger, R. G. Bombardi, J. Govero, Q. Tan, L. A. Durnell, R. N. Coler, M. S. Diamond, J. E. Crowe, S. G. Reed, L. B. Thackray, R. H. Carnahan, N. Van Hoeven. Intramuscular delivery of replicon RNA encoding ZIKV-117 human monoclonal antibody protects against Zika virus infection. Molecular Therapy Methods and Clinical Development. 18, 402–414 (2020). https://doi.org/10.1016/j.omtm.2020.06.011 [↩]
- A. August, H. Z. Attarwala, S. Himansu, S. Kalidindi, S. Lu, R. Pajon, S. Han, J.-M. Lecerf, J. E. Tomassini, M. Hard, L. M. Ptaszek, J. E. Crowe, T. Zaks. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nature Medicine. 27, 2224–2233 (2021). https://doi.org/10.1038/s41591-021-01573-6 [↩]
- J.-Q. Li, Z.-R. Zhang, H.-Q. Zhang, Y.-N. Zhang, X.-Y. Zeng, Q.-Y. Zhang, C.-L. Deng, X.-D. Li, B. Zhang, H.-Q. Ye. Intranasal delivery of replicating mRNA encoding neutralizing antibody against SARS-CoV-2 infection in mice. Signal Transduction and Targeted Therapy. 6, 369 (2021). https://doi.org/10.1038/s41392-021-00783-1 [↩]
- Y.-Q. Deng, N. Zhang, Y. Zhang, Y.-X. Ye, X. Zhang, X. Xue, H. Xu, D.-D. Liu, X.-J. Zhao, Y. Cao, H. Chen, C. Qin, B. Ying. Lipid nanoparticle-encapsulated mRNA antibody provides long-term protection against SARS-CoV-2 in mice and hamsters. Nature, Cell Research. 32, 375–382 (2022). https://doi.org/10.1038/s41422-022-00630-0 [↩]
- D. Vanover, C. Zurla, H. E. Peck, N. Orr‑Burks, J. Y. Joo, J. Murray, N. Holladay, R. A. Hobbs, Y. Jung, L. C. S. Chaves, L. Rotolo, A. W. Lifland, A. K. Olivier, D. Li, K. O. Saunders, G. D. Sempowski, J. E. Crowe Jr., B. F. Haynes, E. R. Lafontaine, R. J. Hogan, P. J. Santangelo. Nebulized mRNA‑encoded antibodies protect hamsters from SARS‑CoV‑2 infection. Advanced Science. 9, e2202771 (2022). https://doi.org/10.1002/advs.202202771 [↩]
- B. Chen, Y. Chen, J. Li, C. Wang, W. Song, Y. Wen, J. Lin, Y. Wu, T. Ying. A single dose of anti-HBsAg antibody-encoding mRNA-LNPs suppressed HBsAg expression: a potential cure of chronic hepatitis B virus infection. mBio. 13(4), e01612-22 (2022). https://doi.org/10.1128/mbio.01612-22 [↩]
- C. E. Deal, A. F. Richards, T. Yeung, M. J. Maron, Z. Wang, Y.-T. Lai, B. R. Fritz, S. Himansu, E. Narayanan, D. Liu, R. Koleva, S. Licht, C. J. Hsiao, I. L. Rajlic, H. Koch, M. Kleyman, M. E. Pulse, W. J. Weiss, J. E. Doering, S. K. Lindberg, N. J. Mantis, A. Carfi, O. J. Plante. An mRNA‑based platform for the delivery of pathogen‑specific IgA into mucosal secretions. Cell Reports Medicine. 4(11), 101253 (2023). https://doi.org/10.1016/j.xcrm.2023.101253 [↩]
- M. Kinoshita, K. Kawaguchi, B. T. Nguyen Le, A. Kainuma, T. Sawa, S. Uchida. Fc-free single chain antibody mRNA therapy for airway infection of multidrug-resistant Pseudomonas aeruginosa. bioRxiv. 659416 (2025). https://doi.org/10.1101/2025.06.12.659416 [↩]
- Y.-S. Wang, M. Kumari, G.-H. Chen, M.-H. Hong, J. P.-Y. Yuan, J.-L. Tsai, H.-C. Wu. mRNA-based vaccines and therapeutics: an in-depth survey of current and upcoming clinical applications. Journal of Biomedical Science. 30, 84 (2023). [↩]
- H.-H. Wei, L. Zheng, Z. Wang. mRNA therapeutics: New vaccination and beyond. Fundamental Research. 3(5), 749–759 (2023). [↩] [↩]






