Abstract
The mitigation of global warming and climate change are significant issues that must be tackled and which will otherwise lead to destruction across the globe. One of the main causes of this is the rampant emission of CO2, making up near 76% of all greenhouse gas emissions. Carbon Capture and Utilization (CCU) is a leading method in combating the rising emissions of CO2. As different methods can change the output and outcome of carbon utilization, various catalysts must be considered to maximize the output. Inorganic catalysts, in particular, stand out as their properties allow for more recyclability and stability over time. For example, metal oxides have shown CO2 adsorption capabilities up to 9.77 mmol/g and enhancement of CO2 conversion to CO by 97% faradaic efficiency. Photocatalysts such as Ru(bpy)₃²⁺ show high CO2 reduction selectivity, and metal organic frameworks such as HKUST-1 showed CO2 capture efficiencies of up to 7.52 mmol/g. Despite these capabilities, problems lie in the cost, scalability, and energy requirements, calling for more technological development. This review will assess the synthesis, capabilities, and applications of metal oxides, photocatalysts, and metal organic frameworks as inorganic catalysts in Carbon utilization processes.
Keywords: Chemistry; Environmental Chemistry; Inorganic Chemistry; Inorganic Catalysts; Carbon Capture Utilization and Storage
Introduction
A direct consequence of burning fossil fuels is the excessive levels of CO2 released, driving global warming and climate change. This generation of CO2 constitutes nearly 76% of global greenhouse gas emissions1. Data collected by NASA show that the past ten years have been the warmest years on record. When compared with 2014, which saw an increase in global temperatures of 0.74 °C relative to a benchmark of the 1950-1981 average, the temperature increase in 2023 was measured at 1.17 °C2. Droughts, floods, heat waves, and other extreme weather events are amplified by this rise in temperature3.It may be difficult, however, to fully “greenify” or electrify specific industries such as oil and gas as they require the powerful capabilities of fossil fuels to produce their energy-intensive processes4. As such, capturing released carbon and repurposing it creates an efficient and sustainable way to slow down the rise in CO2 levels and protect the environment. This strategy, better known as Carbon Capture, Utilization, and Storage (CCUS), is a promising solution.
Through various methods, CCUS can be used to safely and efficiently store emissions, and create products with an already existing market. In particular, methanol and urea are common products of CCUS. Enhanced oil recovery and conversion into biochar are also popular processes, contributing to agriculture and fuel production.
The first step in the CCUS process is carbon capture. As advancements in strategies for CO2 capture have gained increasing global prominence in the past two decades, the technology has favored point sources where CO2 is highly concentrated5. Point-source capture refers to methods in which CO2 is extracted before the release of the other fuel consumption byproducts, namely through pre-combustion, oxyfuel combustion, or direct air capture. However, broader issues across the entire process hinder its viability on an industrial scale.
CCUS processes is currently held back by its developing technology, especially within infrastructure for storing and transporting carbon, as well as a need for sustainable sources of energy6. Due to this, CCUS currently lacks scalability in terms of cost and efficiency. Furthermore, CO2 must undergo purification and transportation prior to utilization, requiring development in those fields alongside utilization for a more cohesive development.
Incorporating catalysts is an essential step in scaling CCUS to an industrial level. As discussed earlier, for CCUS to be improved, other fields such as carbon storage and transport both need to be developed further. As such these inorganic catalysts can assist in CCUS. By introducing catalysts into CCUS processes, they assist with the adsorption and conversion of CO2 into value-added products. In storage, inorganic catalysts can adsorb CO2 through their distinct porous structures and surface modifications. In utilization, catalysts lower the activation energy required to convert the CO2 into useful chemicals. Inorganic catalysts are gaining significant attention for their potential in CO2 capture, owing in part to their intrinsic properties, including porosity and solubility, which may hold significance in creating a greener future. Industrial leaders are already utilizing CO2 and inorganic catalysts in order to create products such as ethylene and polyols, which are then used to replace fossil fuels7’8.
This review focuses specifically on inorganic catalyst. Unlike homogeneous or enzymatic systems, they show capabilities in cost-effectiveness, thermal stability, recyclability, and potential for larger scale application.
Current studies have highlighted various categories and specific catalysts for CCUS. However, few provide a cohesive view and comparison of various inorganic catalysts and their performance. This review will delve into the synthesis, properties, and applications of metal oxides, photocatalysts, and metal-organic frameworks (MOFs) in CCUS, to assess their development, broader applicability, and potential environmental impact.
Methodology
To compile information and data for this review, an extensive search was taken to gather relevant and recent information regarding carbon capture and inorganic catalysis. In particular, sources were chosen from peer-reviewed, high-impact journals from Google Scholar in order to ensure the credibility of the information presented. Sources detailing advancements from the past 20 years were prioritized in order to use the most up-to-date information for the review.
Furthermore, data extraction was conducted with the following keywords: catalysis, CO2 conversion, MOFs in CO2 reduction, photocatalytic CO2 reduction, etc. By using these keywords, sources were narrowed down to be most relevant towards addressing the role of inorganic catalysts in CCUS.
Discussion
Inorganic Catalysts
The relative inertness of CO2 (bond dissociation energy of around 1600 kJ/mol) often requires catalysts to help break the covalent bonds9. Inorganic catalysts show great promise in carbon capture and conversion due to their stability in harsh environments and high turnover numbers. As carbon capture and conversion typically go hand in hand, this section will discuss the development of inorganic catalysts and their applications to both processes.
A common process used in developing inorganic catalysts is sol-gel, a wet chemical method used for the synthesis of nanostructures10. By dissolving a molecular precursor, turning it into a gel, and then drying it, materials such as metal oxides can be synthesized10. Another method is solvothermal synthesis, which is conducted under high pressure and creates high-quality crystalline structures11’12. In this process, a new material is produced by placing a precursor and solvent in a closed system in which the temperature rises beyond the boiling points of the solvent13.
The optimization for certain traits is essential in the synthesis of catalysts. For CCUS, surface area, porosity, and water solubility are critical considerations, as they affect the way the catalysts absorb and adsorb CO2. Catalyst sustainability, either in turnover number or the availability of the material itself, is also an important consideration in maximizing the environmental benefits. Additionally, it is also important to consider the identity and associated properties of the catalyst, which can help maximize efficiency and meet targets, such as environmental impact thresholds. The following sections will examine what properties are associated with various catalysts among metal oxides, photocatalysts, and metal-organic frameworks.
Metal Oxides
A metal oxide is a compound with a metal cation and oxygen anions, mainly in the form of ionic bonds. These have been studied since the 1950s for hydrocarbon processing, in which hydrocarbons are converted into valuable products, and for their oxidizing capabilities14. They are studied so often in part of their Unlike other catalysts, such as zeolites and activated carbons, metal oxides exhibit high thermal stability and high selectivity under harsh conditions15. Additionally, metal oxides’ intrinsic reactivity with CO2 makes them an attractive option for capture and conversion. Furthermore, they are more cost-effective than many catalyst alternatives and are also less toxic16. The reactions catalyzed by metal oxides can be further improved by implementing supporters such as silicas and aluminas, providing better stability for the catalysts17’18. However, a key limitation with widespread metal oxide implementation in CCUS is the high consumption of energy required to activate and in each catalyst turnover, which is correlated with their fast-saturating properties and raises questions of sustainability15’19.
a. Magnesium Oxides
Magnesium oxide (MgO) is particularly useful in performing CO2 absorption, a type of CO2 capture in which CO2 is selectively dissolved from a gas mixture. MgO’s surface morphology is suitable for oxygen generation, resulting in efficient CO2 absorption and low energy consumption for regeneration20. Activating carbon nanofibers with MgO generates a catalyst capable of increasing CO2 absorption capacity up to 2.72 mmol/g21. Supporting activated carbon-based bamboo (BAC) using MgO nanoparticles (NPs) also shows strong adsorption capabilities. Relative to the physical absorption of regular BACs (18.8 mg/g), the MgO(NPs)-BAC (39.8 mg/g) showed a 112% increase in the physical adsorption of CO222. Furthermore, fibrous silicas can be improved using MgO. Regular fibrous silicas show an absorption of CO2 of 0.52 mmol/g, while MgO-infused fibrous silicas have an absorption of 9.77 mmol/g17.
b. Calcium Oxides
Despite excessive sintering and relative ease of decomposition, calcium oxide (CaO) is an excellent solid adsorbent for CO2. CaO nanoparticles derived from nanosized CaCO3 show a 20% increase in the amount of CO2 converted, relative to bulk CaO15. Furthermore, if the CaO is carbonated for a sufficient duration prior to use, the aforementioned drawbacks of sintering and decomposition are significantly reduced23. Another study found that a hydrated solution of CaO in a multi solvent mixture of water and ethanol shows a near 100% increase in the catalyst’s sorption capacity, and it is theorized that alternative syntheses could further increase catalyst efficiency24. CaO, when dispersed on an inactive support such as γ-Al2O3, also demonstrates an increase in sorption capacity25. In comparison to standard CaO, the Al-dispersed CaO showed a higher capacity to bind CO2, reduced sintering, and high efficiency in low temperatures, circumventing a traditional limitation of metal oxide catalysts26. The dispersed CaO also shows long-term stability, maintaining 90% of its efficiency as compared to bulk CaO (at 50%) after 20 cycles26.
c. Zinc Oxides
Five metal oxides (ZnO, SnO2, Fe2O3, La2O3, and CeO2) were tested to see which would have the greatest catalytic effect on the process of transforming glycerol into glycerol carbonate with CO2 as a reactant (Figure 1)27. Zinc oxide showed the greatest efficiency, yielding 8.1% of glycerol carbonate28. Furthermore, bimetallic Zn–Cu electrocatalysts can be used to selectively achieve a highly efficient conversion of CO₂ into CO. While the selectivity of ZnO alone is limited to 30% faradaic efficiency, a measurement of how efficiency charges are transferred in a system during an electrochemical reaction, the two-metal combination increases the selectivity to 97% faradaic efficiency28. Additionally, a CuO-ZnO-ZrO₂ catalyst supported by graphene oxide (GO) has shown excellent efficiency in converting CO2 to methanol. The addition of GO (0.5-2.5 wt%) increases the availability of active sites for the adsorption CO2 and H2, thus increasing selectivity for methanol production from around 70% (without GO) to 75.9% (with GO) and further enhancing the overall yield of methanol29.
d. Nickel
Nickel oxides (NiO) are attractive metal complexes for Carbon utilization, with high reactivity and cost-efficiency. However, drawbacks include their tendency to sintering at high temperatures and deactivation from coke formation30. Ceria has been shown to counteract these limitations by dispersing the NiO over a support framework and reducing sintering, thus enhancing the Ni’s performance31. Reduced GO-supported NiO shows a 63.1% CO2 conversion rate, while the addition of ceria increases the rate to 84.5%32. NiO nanoparticles themselves are also competent at carbon capture, increasing carbon absorption by 34% in limited-mixing conditions (incompletely mixed) and 54% in high-mixing conditions (completely mixed)15.
e. Titanium
TiO2, a porous metal oxide, shows a rate of catalytic conversion of CO2 up to 10 times higher than methane’s, yielding from 2 to 3 times as much CO33. A bimetallic combination of bismuth and titanium (Bi2O3-TiO2) showed that TiO2 polymorphs such as rutile and anatase can be modified with this compound to better adsorb CO234. Carboxylate production from CO2 through electron transfer is also made possible through the catalyst’s strong adsorption and alteration capabilities34. Thus, TiO2 is a versatile stepping stone to the reductive conversion of CO2 to other value-added materials.
Try to be conscious of your figure placement. Figure 1 is plopped right in the middle of your discussion. It would make more sense to have it right at the beginning, or at the end of your Metal Oxides discussion; not in the middle.
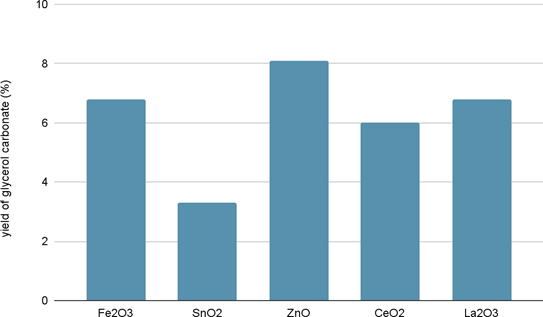
| Lifetime | Cost (per 100 grams) | |
| Magnesium Oxide | Lasts around 10-20 cycles15 | ~$85 per 100 grams35 |
| Calcium Oxide | Lasts over 20 cycles (when dispersed on γ-Al₂O₃.)15 | ~$9 per 100 grams36 |
| Zinc Oxide | Lasts over 25 adsorption–desorption cycles37 | ~50$ per 100 grams38 |
| Nickel Oxide | Doesn’t last long, but its lifetime can be extended with ceria as a supporter15 | ~$140 per 100 grams39 |
| Titanium Oxide | Lasts incredibly long even in sunlight40 | ~$60 per 100 grams41 |
This overview provides a comparison of metal oxides’ cost and lifetime in addition to their reactivity and efficiencies presented earlier.
Photocatalysts
Unlike metal oxides, which use heat as a source of activation, photocatalysts are compounds that absorb photons as a means to gather energy and perform redox reactions and/or sensitizations. As a result, photocatalysts provide a more environmentally positive option for CO2 transformation, gaining attention in recent years as their loadings tend to be significantly lower than those of traditional catalysts. Furthermore, as they are activated by light, they do not require harsh or energetically costly conditions (e.g. high temperatures). Solar energy is also a major source of photons, allowing photochemical transformation through this method to have minimal impact on the environment33. Furthermore, spatiotemporal control can be achieved with the use of photocatalysis, as the scope of reactivity is localized to the presence of both the light and the catalyst42. Photocatalysts are also capable of creating exotic and valuable bond constructions more readily than previously established protocols43. However, a clear disadvantage of using photocatalysts is that they can lack substrate selectivity, leading to off-cycle reactivity and low CO2 reduction33. As such, a heavy emphasis in photocatalyst development for CO2 capture and conversion is on improving yields of and selectivity for the desired product.
a. Re(I)
A commonly utilized metal complex in CO2 conversion is rhenium(I), which displays high selectivity of products (preferentially forming CO) and reduction efficiency of CO244. Rhenium can be utilized through Re(bpy)(CO)3L complexes, where L is an X-type ligand, typically -NCS, -Cl, or -CN44. The NCS complex is the most efficient, producing around 60 μmol of CO after 25 hours of irradiation, while the -Cl complex produces half of that amount44. All three examples also show the highest efficiency between 300 and 400 nm (UV) irradiation44. In a DMF-triethanolamine solvent system, the production efficiency and selectivity of CO are increased45. Furthermore, adding bromide or chloride counterions can increase the durability of the complex. This prevents the formation of formate complexes, which can lower the CO2 reduction efficiency45. However, one significant drawback of widespread Re(I) adoption is the toxicity associated with the CO ligands. Further development and addressing this issue may make Re(I) complexes even more prominent.
b. (GO)-TiO2-Ag2O (with or without Arg)
GO, when combined with TiO2, a semiconductor used for CO2 reduction, possesses considerable absorption and a large conductivity capacity (∼5000 W m-1 K-1), creating a versatile composite46. Adding silver oxide (Ag2O) allows the catalyst to surpass TiO2’s catalytic limitations47. The energy gap between this photoactive composite’s ground and excited states can be bridged with high-energy UV light with excellent efficiency47. Adding arginine (Arg) as a sacrificial agent increases the catalyst’s overall efficiency and the photoluminescence (PL) absorption surface, red-shifting its absorbance wavelength to the visible light region47. GO-TiO2-Ag2O-Arg shows higher absorption of CO2 than GO-TiO2-Ag2O under most conditions, aside from irradiation under UV light at 40 °C, where GO-TiO2-Ag2O outperforms the Arg-modified catalyst by about 300 mmol/g (Table 1)47. Under UV and visible light, respectively, GO-TiO2-Ag2O produces 32.616 μmol/gcatalyst of methanol, and GO-TiO2-Ag2O-Arg produces 20.385 μmol/gcatalyst of methanol over 4 hours, outperforming the yields of comparable photocatalysts (Table 2)47’48’49’50’51’52’53’54’55. When reused, both composites lose little efficiency, suggesting that they may be sustainably viable47.
c. Ru(bpy)
The photocatalyst [Ru(bpy)(CO)₂Cl₂] (and its reduced form, [Ru(bpy)(CO)₂]2+) excels at the conversion of CO2 into CO and formic acid via a multi-step photo-electrocatalytic cycle. When combined with a nitrogen doped Ta2O5 semiconductor, the complex acts as a charge-transfer mediator between the photosensitizer and CO2. In these conditions, its performance increases dramatically, showing turnover numbers less than 10 as well as a redox potential of −0.7 V vs. SHE56. When in contact with light, electrons from the semiconductor are transferred to the Ru-complex. Through sequential two-electron reduction, the hydride complex [Ru(bpy)2(CO)H]+ is created, which takes in CO2, and eventually creates formic acid, after which the cycle renews56. With a free energy of −18.56 kcal mol-1 and an activation energy of +32.91 kcal mol-1, the cycle is accessible both kinetically and thermodynamically56. The selective product formation and absorption of light makes Ru- based complexes promising for CO2 conversion. However, as its precursor utilizes a rare metal and released carbon monoxide in its cycle, its potential usage is hindered by its economical viability and environmental impact56.
| Catalyst | Temp (°c) | Light | CO2 absorption (mmol/g) | |
| 1 | GO-TiO2-Ag2O-Arg | r.t. | UV | 1237.815 |
| 40 | UV | 690.27 | ||
| r.t. | visible | 1255.461 | ||
| 40 | visible | 786.544 | ||
| 2 | GO-TiO2-Ag2O | r.t. | UV | 1209.27 |
| 40 | UV | 988.695 | ||
| r.t. | visible | 1183.32 | ||
| 40 | visible | 621.502 |
conditions (10 bar)47.
This table compares the CO2 absorption efficiencies of various photocatalysts under different light conditions.
| Catalyst (g) | Reaction condition light / time (hr) | Product (μmol/g catalyst) | |
| 1 | V-TiO2 (0.2) | Visible/4 | CH3OH (4.6) |
| Cr-TiO2 (0.2) | CH3OH (2.94) | ||
| Co-TiO2 (0.2) | CH3OH (6.53) | ||
| 2 | Ag-TiO2 (0.1) | UV–Visible/8 and 6 | CH3OH (29) |
| CH3OH (15) | |||
| 3 | N-doped TiO2 (0.6) | UV–Visible/2 | CH3OH (20) |
| 4 | N–TiO2 (0.1) | Visible/2 | CH3OH (0.2) |
| 5 | FeTiO3/TiO2 (0.05) | UV–Visible/3 | CH3OH (1.386–1.296) |
| 6 | Cu/Fe–TiO2-SiO2 | no hν | CH3OH (4.12) |
| 7 | Ti-silica film | no hν | CH3OH (11) |
| 8 | g-C3N3 (0.1) | UV–Visible/1 | CH4/CH3OH (0.26) |
| amine-functionalized g-C3N4 (0.1) | UV–Visible/1 | CH3OH (0.28) | |
| 9 | GO-TiO2-Ag2O (0.1) | UV/4 | CH3OH (32.616) |
| 10 | GO-TiO2-Ag2O-Arg (0.1) | Visible/4 | CH3OH (20.385) |
This table compares the methanol production efficiencies of various photocatalysts under different light conditions.
| Lifetime | Cost | |
| Re(I) | TON of 30 (with 1-NCS)45 | ~$500 per gram (precursor)58 |
| (GO)-TiO2-Ag2O | Can be reused many times without losing efficiency47 | ~$60 per 100 grams41 |
| Ru(bpy)32+ | TON of 4000 (with 5.0 μM of trans(Cl)–Ru(bpy)(CO)₂Cl₂)59 | ~$278 per gram60 |
This overview provides a comparison of photocatalysts’ cost and lifetime in addition to their reactivity and efficiencies presented earlier.
Metal-Organic Frameworks
Metal-organic frameworks (MOFs) are marked by their highly tunable, lattice-like structures composed of metal nodes and organic linkers, and large surface area, setting them apart from metal oxides and photocatalysts. Furthermore, MOFs contain an extremely high degree of porosity, making them an attractive option for CO2 capture61. MOFs are also stable, maintaining structural integrity over multiple cycles of use, which contributes to arguments for their sustainability and scalability62. Their main advantage over other catalysts arises from their ability to be modularized both before and after synthesis. As a result, many of MOFs’ structural properties, such as pore size, metal centers, and organic linkers, can be tuned for a specific purpose63. However, the reaction environment may significantly influence their performance and reactivity. Moisture, for example, can decompose the MOFs64. They are also costly to produce on large scale, and as such, scalability to industrial levels is a significant challenge to be addressed. Despite these limitations, MOFs show potential for their long-lasting lifetime and CCS/CCU efficiency.
a. MOF-808
MOF-808 is composed of hydroxo/aquo-termimated Zr6O8 clusters held together by benzene-1,3,5-tricarboxilate (BTC) ligands65. By binding amino acids to the backbone, different functionalities and qualities of MOF-808 derivatives, such as their regeneration and CO2 capture efficiency, were tested. Among these amino acids, MOF-808 absorbed the most CO2 when combined with glycine (15 kPa; 0.693 mmol/g) and DL-lysine (15 kPa; 1.949 mmol/g)66. MOF-808 also possesses interconnected pores of varying sizes, consisting of smaller pores (which are inaccessible to guest molecules) and complementary larger pores, contributing to the selectivity for CO267. The amino groups on alkyl chains are oriented towards these pores, which are the primary sites for CO2 capture67.
A vital source of CO2 is flue gas, which is constituted mainly by CO2 (8-15%)68. In order to capture flue gas at a low cost, MOFs need to be moderately water and moisture-tolerant. MOF-808s have shown retained catalytic behavior under humid conditions, with certain variations such as MOF-808-Gly even enabling an increased CO2 uptake when in humid conditions67. This makes MOF-808 more efficient in an environment where other catalysts may deteriorate, maintaining uptake capacity even after 80 cycles66. This implies its sustainability for long-term and widespread usage, as it can operate effectively within a green solvent (i.e. water).
b. MOF-5
MOF-5 is distinguished by its exceptionally stable and porous framework. Its structure is defined by an octahedral array of 1,4-benzene-dicarboxylate (BDC) groups joined with inorganic [OZn4]6+ groups69. Even when solvent molecules are evacuated, MOF-5s maintain their structure and porosity, emphasizing their potential in CO2 storage61. MOF-5s also contain Zn2+, which harbor defect sites (i.e. crystalline imperfections) that can serve as the active sites for the reaction of CO2 to epoxides, forming the matching cyclic carbonate70. However, this reaction operates mainly under harsh conditions, requiring high temperatures and pressures61. This suggests that the catalytic activity within defect sites may not produce enough yield to be considered efficient. As such, research aimed towards increasing the density of defect sites is an active area of exploration. Methods such as post-synthetic treatment with acids and bases, as well as using isostructural mixed linkers, have also been explored to increase the performance of MOF-5 and its derivatives71.
c. HKUST-1
HKUST-1, made of copper nodes and connected by benzene-1,3,5-tricarboxilate (BTC) ligands, is characterized by a large surface area and porous structure, characteristic of efficient MOF-based catalysts72. Its structure contains linked cages, one of smaller diameter (3.5 Å) and one larger (9 Å)73. The main appeal of HKUST-1 stems from the benefits conveyed by post-synthetic modification using amines. By heating the molecule at 200 °C, water is removed, leaving behind coordinatively unsaturated sites (CUS)73. Amines, which have a high CO2 capture yield, are then attached to the CUSs. For instance, Zelenka and colleagues used ethylenediamine (en) and diethylenetriamine (deta) to modify the catalyst73. By introducing these amines at different ratios relative to the core MOF structure, they determined which amine loadings result in the greatest yields. At higher MOF to amine ratios (1:2 for en, 1:1.15 for deta), the compound shows signs of decomposing due to the alkaline-dense environment73. Further amine loading screens indicate that lower MOF-to-amine ratios are favorable for increasing CO2 capture73. With en, the highest yield of captured CO2 is 22.31 wt.% (5.07 mmol/g), at a MOF to amine ratio of 1:0.173. With deta, the MOF yields 33.09 wt.% of captured CO2 (7.52 mmol/g), at 1:0.0573. Increasing the ligand size in a HKUST-1 with a copper center shows a decrease in copper density74. While this lowers the MOF’s conductivity, it contributes to greater versatility in gas storage74.
| Lifetime | Cost | |
| MOF-808 | Lasts over 80 vacuum swing adsorption cycles67 | ~$866 per kilogram75 |
| MOF-5 | Lasts over 50 adsorption-desorption cycles76 | ~$530 per kilogram77 (78.6% of the cost is the solvent) |
| HKUST-1 | Lasts over 10 adsorption-desorption cycles78 | ~$70 per gram79 |
This overview provides a comparison of MOFs’ cost and lifetime in addition to their reactivity and efficiencies presented earlier.
Challenges and limitations
While inorganic catalysts provide hope in making CCUS more sustainable and viable, the scalability of both the CCUS processes and catalysts remain a large issue. High costs, material scarcity, and threats to the environment stunt the field from growing into an industry-scale process.
Catalysts can be used to make these processes faster, but also suffer from largely the same limitations as conventional methods of CCUS. For example, while Re- and Ru-based photocatalysts have powerful reduction capabilities, they often require expensive and scarce metal precursors (0.7 and 1 ppb in the crust of the earth, respectively)80. The scarcity and cost of materials make them hard to bring into an industry scale. Using photocatalysts also comes with dangers. [Ru(bpy)(CO)₂Cl₂], contain and release CO, which is highly toxic and hence dangerous to scale up. Furthermore, photocatalysts typically use organic solvents such as DMF, which can only be disposed through storage or burning. While this is passable in a laboratory setting, it’s another factor to consider in scaling up catalysts.
CCUS is a great step towards cutting down on carbon-emissions, however, as seen by the review, these processes and catalysts typically have a high energy cost, potentially making it less sustainable. Without advancements in catalyst efficiency and renewable energy sources, this process may leave harmful footprints behind its intended purpose of cleaning the environment.
Conclusion
Within CCUS, inorganic catalysts may be a potential solution due to their outstanding efficiency and properties. Three categories of these catalysts have been explored in recent research efforts and are highlighted in this review: metal oxides, photocatalysts, and MOFs.
Metal oxides, including magnesium, calcium, zinc, nickel, and titanium oxides, are highlighted for their strong adsorption capabilities and reactivity with CO2. However, some suffer from deactivation and sintering, requiring extra supports to fully utilize their properties. Furthermore, photocatalysts, such as Re(I), (GO)-TiO2-Ag2O, and Ru(bpy)32+, all exhibit highly versatile reactivity alongside their standout ability to utilize solar power. More development and optimization of their properties may modify these catalysts to be more efficient in CCUS. Finally, MOFs, including MOF-808, MOF-5, and HKUST-1, demonstrate excellent modularity and structural efficiency for CCUS. However, they are currently hampered in their high cost and inability to withstand certain reaction conditions.
This technology is still in its early stages of development and requires efficiency, cost, and scalability development. These critical factors must be addressed before the employment of inorganic catalysts in CCUS becomes more widespread. Strides are being taken outside of CCUS to help approach the net zero emissions goal (NZE) by 2050, including growth in sales of electric vehicles and promotion of energy efficiency81. However, most of these goals are currently behind target benchmarks and require more intensive efforts to achieve widespread adoption. CCU is one such goal, indicating a call for greater urgency of implementation if the NZE goal is to be achieved. Projections indicate that a successful integration of CCUS technologies may mitigate up to 25.4% of total carbon emissions2. Increasing efforts are being invested in the development of these CCUS technologies, and there is hope that CCUS will enable a significant and positive change to how our global society is powered.
References
- Center for Climate and Energy Solutions. Global Emissions. https://www.c2es.org/content/international-emissions/#:~:text=by%20Gas%2C%202015-,Not es,are%20expressed%20in%20CO2-equivalents. (accessed 2024-09-04). [↩]
- Power Technology. How is global industry utiliing CCUS technologies in 2024? https://www.power-technology.com/features/how-is-global-industry-utilising-ccus-technologie s-in-2024/ (accessed 2024-09-04). [↩] [↩]
- Global Temperature. https://climate.nasa.gov/vital-signs/global-temperature/?intent=121 (accessed 2024-09-04). [↩]
- Extreme Weather and Climate Change. https://science.nasa.gov/climate-change/extreme-weather/ [↩]
- K. Thambimuthu, M. Soltanieh, J. C. Abanades. In Carbon Dioxide Capture and Storage; Special Report of the Intergovernmental Panel on Climate Change; Metz, B., Ed.; Cambridge University Press, 105–178 (2025). [↩]
- M. J. Regufe, A. Pereira, A. F. P. Ferreira, A. M. Ribeiro, A. E. Rodrigues. Current Developments of Carbon Capture Storage and/or Utilization–Looking for Net-Zero Emissions Defined in the Paris Agreement. Energies. 14, 2406 (2014). [↩]
- LanzaTech. LanzaTech Produces Ethylene from CO2, Changing the Way We Make Products Today. https://lanzatech.com/lanzatech-produces-ethylene-from-co2-changing-the-way-we-make-pr oducts-today/ [↩]
- Covestro. CO₂ as a new raw material – becoming a jack of all trades. https://www.covestro.com/en/sustainability/what-drives-us/circular-economy/alternative-reso urces/co2-as-a-raw-material [↩]
- Minyukova, T. P.; Dokuchits, E. V. Hydrogen for CO2 Processing in Heterogeneous Catalytic Reactions. Int. J. Hydrog. Energy. 48, 22462–22483 (2023). [↩]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I. H.; Valiev, G. H.; Kianfar, E. Nanomaterial by Sol‐gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021 (2021). [↩] [↩]
- González, C. M.; Morales, E. M.; Tellez, A. de; Quezada, T. E.; Kharissova, O. V.; Méndez-Rojas, M. A. CO2 Capture by Mofs. Handb. Greener Synth. Nanomater. Compd. 2, 407–448 (2021). [↩]
- Zhong, H.; Mirkovic, T.; Scholes, G. D. Nanocrystal Synthesis. Compr. Nanosci. Nanotechnol. 1-5, 153–201 (2011). [↩]
- Ledoux, G.; Joubert, M. F.; Mishra, S. Upconversion Phenomena in Nanofluorides. Photonic Electron. Prop. Fluoride Mater. 35–63 (2016). [↩]
- Laurent, S.; Boutry, S.; Muller, R. N. Metal Oxide Particles and Their Prospects for Applications. Iron Oxide Nanopart. Biomed. Appl. 3–42 (2018). [↩]
- Khdary, N.H.; Alayyar, A.S.; Alsarhan, L.M.; Alshihri, S.; Mokhtar, M. Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review. Catalysts. 12, 300 (2022). [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Azmi, A. A.; Ruhaimi, A. H.; Aziz, M. A. A. Efficient 3-Aminopropyltrimethoxysilane Functionalised Mesoporous Ceria Nanoparticles for CO2 Capture. Mater. Today Chem. 16, 100273 (2016). [↩]
- Yusof, S. M.; Othaman, R.; Setiabudi, H. D.; Teh, L. P. Modified Fibrous Silica for Enhanced Carbon Dioxide Adsorption: Role of Metal Oxides on Physicochemical Properties and Adsorption Performance. J. Solid State Chem. 294, 121845 (2021). [↩] [↩]
- Alrafei, B.; Polaert, I.; Ledoux, A.; Azzolina-Jury, F. Remarkably Stable and Efficient Ni and Ni-Co Catalysts for CO2 Methanation. Catal. Today 346, 23–33 (2020). DOI:10.1016/j.cattod.2019.03.026. [↩]
- Hashemi, S. M.; Karami, D.; Mahinpey, N. Solution Combustion Synthesis of Zirconia-Stabilized Calcium Oxide Sorbents for CO2 Capture. Fuel 269, 117432 (2020). DOI:10.1016/j.fuel.2020.117432. [↩]
- Li, P.; Chen, R.; Lin, Y.; Li, W. General Approach to Facile Synthesis of Mgo-Based Porous Ultrathin Nanosheets Enabling High-Efficiency CO2 Capture. Chem. Eng. J. 404, 126459 (2021). DOI:10.1016/j.cej.2020.126459. [↩]
- Othman, F. E.; Yusof, N.; Samitsu, S.; Abdullah, N.; Hamid, M. F.; Nagai, K.; Abidin, M. N.; Azali, M. A.; Ismail, A. F.; Jaafar, J.; Aziz, F.; Salleh, W. N. Activated Carbon Nanofibers Incorporated Metal Oxides for CO2 Adsorption: Effects of Different Type of Metal Oxides. J. CO2 Util. 45, 101434 (2021). [↩]
- Wan Isahak, W. N.; Ramli, Z. A.; Mohamed Hisham, M. W.; Yarmo, M. A. Magnesium Oxide Nanoparticles on Green Activated Carbon as Efficient CO2 Adsorbent. AIP Conf. Proc. 1571, 882-889 (2013). [↩]
- Florin, N. H.; Harris, A. T. Reactivity of Cao Derived from Nano-Sized CACO3 Particles through Multiple CO2 Capture-and-Release Cycles. Chem. Eng. Sci. 64, 187–191 (2009). [↩]
- Li, Y. ‐J.; Zhao, C. ‐S.; Qu, C. ‐R.; Duan, L. ‐B.; Li, Q. ‐Z.; Liang, C. CO2 Capture Using CaO Modified with Ethanol/Water Solution during Cyclic Calcination/Carbonation. Chem. Eng. Technol. 31, 237–244 (2008). DOI:10.1002/ceat.200700371. [↩]
- Belova, A. A.; Yegulalp, T. M.; Yee, C. T. Feasibility Study of in Situ CO2 Capture on an Integrated Catalytic CO2 Sorbent for Hydrogen Production from Methane. Energy Procedia 1, 749–755 (2009). [↩]
- Duyar, M. S.; Farrauto, R. J.; Castaldi, M. J.; Yegulalp, T. M. In Situ CO2 Capture Using CaO/γ-Al2O3 Washcoated Monoliths for Sorption Enhanced Water Gas Shift Reaction. Ind. Eng. Chem. Res. 53, 1064–1072 (2013). [↩] [↩]
- Ozorio, L. P.; Mota, C. J. Direct Carbonation of Glycerol with CO2 Catalyzed by Metal Oxides. ChemPhysChem 18, 3260–3265 (2017). [↩] [↩]
- Wang, L.; Peng, H.; Lamaison, S.; Qi, Z.; Koshy, D. M.; Stevens, M. B.; Wakerley, D.; Zamora Zeledón, J. A.; King, L. A.; Zhou, L.; Lai, Y.; Fontecave, M.; Gregoire, J.; Abild-Pedersen, F.; Jaramillo, T. F.; Hahn, C. Bimetallic Effects on Zn-Cu Electrocatalysts Enhance Activity and Selectivity for the Conversion of CO2 to Co. Chem Catal. 1, 663–680 (2021). [↩] [↩]
- Witoon, T.; Numpilai, T.; Phongamwong, T.; Donphai, W.; Boonyuen, C.; Warakulwit, C.; Chareonpanich, M.; Limtrakul, J. Enhanced Activity, Selectivity and Stability of a Cuo-Zno-Zro2 Catalyst by Adding Graphene Oxide for CO2 Hydrogenation to Methanol. Chem Eng J. 334, 1781–1791 (2018). [↩]
- Zhang, R.; Lu, K.; Zong, L.; Tong, S.; Wang, X.; Zhou, J.; Lu, Z.-H.; Feng, G. Control Synthesis of CEO2 Nanomaterials Supported Gold for Catalytic Oxidation of Carbon Monoxide. Mol. Catal. 442, 173–180 (2017). [↩]
- Qin, Z.; Ren, J.; Miao, M.; Li, Z.; Lin, J.; Xie, K. The Catalytic Methanation of Coke Oven Gas over Ni-Ce/Al2o3 Catalysts Prepared by Microwave Heating: Effect of Amorphous Nio Formation. Appl. Catal. B: Environ. 2015, 164, 18–30 (2015). [↩]
- Hu, F.; Tong, S.; Lu, K.; Chen, C.-M.; Su, F.-Y.; Zhou, J.; Lu, Z.-H.; Wang, X.; Feng, G.; Zhang, R. Reduced Graphene Oxide Supported Ni-Ce Catalysts for CO2 Methanation: The Support and Ceria Promotion Effects. J. CO2 Util. 34, 676–687 (2019). [↩]
- Yaashikaa, P. R.; Senthil Kumar, P.; Varjani, S. J.; Saravanan, A. A Review on Photochemical, Biochemical and Electrochemical Transformation of CO2 into Value-Added Products. J. CO2 Util. 33, 131–147 (2019). [↩] [↩] [↩]
- Nolan, M. Adsorption of CO2 on Heterostructures of Bi2O3 Nanocluster-Modified TiO2 and the Role of Reduction in Promoting CO2 Activation. ACS Omega. 3, 13117–13128 (2018). [↩] [↩]
- https://www.fishersci.com/shop/products/magnesium-oxide-heavy-powder-usp-fcc-fisher-che mical-2/M68500 [↩]
- Lab Alley. Calcium Oxide Powder, Lab Grade.https://www.laballey.com/products/calcium-oxide-powder-lab-grade [↩]
- Pełech, I.; Sibera, D.; Staciwa, P.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Wanag, A.; Narkiewicz, U.; Morawski, A.W. ZnO/Carbon Spheres with Excellent Regenerability for Post-Combustion CO2 Capture. Materials 2021, 14, 6478. https://doi.org/10.3390/ma14216478 [↩]
- Sigma-Aldrich. Zinc Oxide, ReagentPlus®, Powder, <5 μm Particle Size, 99.9%. https://www.sigmaaldrich.com/US/en/product/sigald/205532 [↩]
- Sigma-Aldrich. Nickel(II) Oxide, 99.99% Trace Metals Basis. https://www.sigmaaldrich.com/US/en/product/aldrich/203882 [↩]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Influence of Titanium Dioxide Particle Size on the Photostability of the Chemical UV-Filters Butyl Methoxy Dibenzoylmethane and Octocrylene in a Microemulsion. Cosmetics 2014, 1, 128-139. https://doi.org/10.3390/cosmetics1020128 [↩]
- Sigma-Aldrich. Titanium(IV) Oxide, Anatase, Powder, 99.8% Trace Metals Basis. https://www.sigmaaldrich.com/US/en/product/aldrich/232033 [↩] [↩]
- Deng, S.; Jolly, B. J.; Wilkes, J. R.; Mu, Y.; Byers, J. A.; Do, L. H.; Miller, A. J.; Wang, D.; Liu, C.; Diaconescu, P. L. Spatiotemporal Control for Integrated Catalysis. Nat. Rev. Methods Primers. 3, 28 (2023). [↩]
- Prier, C. K.; Rankic, D. A.; MacMillan, D. W. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 113, 5322–5363 (2013). [↩]
- Takeda, H.; Koike, K.; Inoue, H.; Ishitani, O. Development of an Efficient Photocatalytic System for CO2 Reduction Using Rhenium(I) Complexes Based on Mechanistic Studies. J. Am. Chem. Soc. 130, 2023–2031 (2008). [↩] [↩] [↩] [↩]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Efficient Photochemical Reduction of CO2 to CO by Visible Light Irradiation of Systems Containing Re(Bipy)(CO)3X or Ru(Bipy)32+–Co2+ Combinations as Homogeneous Catalysts. J. Chem. Soc., Chem. Commun. 9, 536–538 (1983). [↩] [↩] [↩]
- Padmanabhan, N. T.; Thomas, N.; Louis, J.; Mathew, D. T.; Ganguly, P.; John, H.; Pillai, S. C. Graphene Coupled TIO2 Photocatalysts for Environmental Applications: A Review. Chemosphere 271, 129506 (2021). [↩]
- Nosrati, A.; Javanshir, S.; Feyzi, F.; Amirnejat, S. Effective CO2 Capture and Selective Photocatalytic Conversion into CH3OH by Hierarchical Nanostructured GO–TiO2–Ag2O and GO–TiO2–Ag2O–Arg. ACS Omega 8, 3981–3991 (2023). [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Ola, O.; Maroto-Valer, M. M. Transition Metal Oxide Based Tio2 Nanoparticles for Visible Light Induced CO2 Photoreduction. Appl. Catal. A: Gen. 502, 114–121 (2015). [↩] [↩]
- Yu, B.; Zhou, Y.; Li, P.; Tu, W.; Li, P.; Tang, L.; Ye, J.; Zou, Z. Photocatalytic Reduction of CO2over Ag/TiO2 Nanocomposites Prepared with a Simple and Rapid Silver Mirror Method. Nanoscale. 8, 11870–11874 (2016). [↩] [↩]
- Michalkiewicz, B.; Majewska, J.; Kądziołka, G.; Bubacz, K.; Mozia, S.; Morawski, A. W. Reduction of CO2 by Adsorption and Reaction on Surface of Tio2-Nitrogen Modified Photocatalyst. J. CO2 Util. 5, 47–52 (2014). [↩] [↩]
- Akple, M. S.; Low, J.; Qin, Z.; Wageh, S.; Al-Ghamdi, Ahmed. A.; Yu, J.; Liu, S. Nitrogen-Doped Tio2 Microsheets with Enhanced Visible Light Photocatalytic Activity for CO2 Reduction. Chinese J. Catal. 36 2127–2134 (2015). [↩] [↩]
- Truong, Q. D.; Liu, J.-Y.; Chung, C.-C.; Ling, Y.-C. Photocatalytic Reduction of CO2 on FETIO3/Tio2 Photocatalyst. Catal. Commun. 19, 85–89 (2012). [↩] [↩]
- Wu, J. C. Photocatalytic Reduction of Greenhouse Gas CO2 to Fuel. Catal. Surv. Asia. 13, 30–40 (2009). [↩] [↩]
- Jacob-Lopes, E.; Revah, S.; Hernández, S.; Shirai, K.; Franco, T. T. Development of Operational Strategies to Remove Carbon Dioxide in Photobioreactors. Chem Eng J. 153, 120–126 (2009). [↩] [↩]
- Huang, Q.; Yu, J.; Cao, S.; Cui, C.; Cheng, B. Efficient Photocatalytic Reduction of CO2 by Amine-Functionalized G-C3n4. Appl. Surf. Sci. 358, 350–355 (2015) [↩]
- Damas, G. B.; Ivashchenko, D. A.; Rivalta, I.; Araujo, C. M. Carbon Dioxide Reduction Mechanism on Ru-Based Electrocatalysts [Ru(bpy)₂(CO)₂]²⁺: Insights from First-Principles Theory. Sustain. Energy Fuels 5, 6066–6076 (2021). [↩] [↩] [↩] [↩]
- Huang, Q.; Yu, J.; Cao, S.; Cui, C.; Cheng, B. Efficient Photocatalytic Reduction of CO2 by Amine-Functionalized G-C3n4. Appl. Surf. Sci. 358, 350–355 (2015 [↩]
- Sigma-Aldrich. Rhenium(III) chloride, powder, 61.4–65.9% Re.https://www.sigmaaldrich.com/US/en/product/aldrich/309184 [↩]
- Kuramochi, Y.; Itabashi, J.; Fukaya, K.; Enomoto, A.; Yoshida, M.; Ishida, H. Unexpected Effect of Catalyst Concentration on Photochemical CO₂ Reduction by trans(Cl)–Ru(bpy)(CO)₂Cl₂: New Mechanistic Insight into the CO/HCOO⁻ Selectivity. Chem. Sci. 6, 3063–3074 (2015). [↩]
- Sigma-Aldrich. Ruthenium powder, 99.99% trace metals basis. https://www.sigmaaldrich.com/US/en/product/aldrich/545023 [↩]
- Olajire, A. A. Synthesis Chemistry of Metal-Organic Frameworks for CO 2 Capture and Conversion for Sustainable Energy Future. Renew. Sustain. Energy Rev. 92, 570–607 (2018). [↩] [↩] [↩]
- Sumida, K.; Rogow, D. L.; Mason, J. A.; McDonald, T. M.; Bloch, E. D.; Herm, Z. R.; Bae, T.-H.; Long, J. R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 112, 724–781 (2011). [↩]
- Olajire, A. A. Synthesis Chemistry of Metal-Organic Frameworks for CO2 Capture and Conversion for Sustainable Energy Future. Renew. Sustain. Energy Rev. 92, 570–607 (2018). [↩]
- An, Y.; Lv, X.; Jiang, W.; Wang, L.; Shi, Y.; Hang, X.; Pang, H. The Stability of Mofs in Aqueous Solutions—Research Progress and Prospects. Green Chem. Eng. 5, 187–204 (2024). [↩]
- del Castillo-Velilla, I.; Sousaraei, A.; Romero-Muñiz, I.; Castillo-Blas, C.; S. J. Méndez, A.; Oropeza, F. E.; de la Peña O’Shea, V. A.; Cabanillas-González, J.; Mavrandonakis, A.; Platero-Prats, A. E. Synergistic Binding Sites in a Metal-Organic Framework for the Optical Sensing of Nitrogen Dioxide. Nat. Commun 14, 2506, (2023). [↩]
- Lyu, H.; Chen, O. I.-F.; Hanikel, N.; Hossain, M. I.; Flaig, R. W.; Pei, X.; Amin, A.; Doherty, M. D.; Impastato, R. K.; Glover, T. G.; Moore, D. R.; Yaghi, O. M. Carbon Dioxide Capture Chemistry of Amino Acid Functionalized Metal–Organic Frameworks in Humid Flue Gas. J. Am. Chem. Soc. 144, 2387–2396 (2022). [↩] [↩]
- Lyu, H.; Chen, O. I.-F.; Hanikel, N.; Hossain, M. I.; Flaig, R. W.; Pei, X.; Amin, A.; Doherty, M. D.; Impastato, R. K.; Glover, T. G.; Moore, D. R.; Yaghi, O. M. Carbon Dioxide Capture Chemistry of Amino Acid Functionalized Metal–Organic Frameworks in Humid Flue Gas. J. Am. Chem. Soc. 144, 2387–2396 (2022). [↩] [↩] [↩] [↩]
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 1–34 (2014). [↩]
- Rosi, N. L.; Eckert, J.; Eddaoudi, M.; Vodak, D. T.; Kim, J.; O’Keeffe, M.; Yaghi, O. M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 300, 1127–1129 (2003). [↩]
- Beyzavi, M. H.; Stephenson, C. J.; Liu, Y.; Karagiaridi, O.; Hupp, J. T.; Farha, O. K. Metal–Organic Framework-Based Catalysts: Chemical Fixation of CO2 with Epoxides Leading to Cyclic Organic Carbonates. Front. Energy Res. 2, (2015). [↩]
- Ravon, U.; Savonnet, M.; Aguado, S.; Domine, M. E.; Janneau, E.; Farrusseng, D. Engineering of Coordination Polymers for Shape Selective Alkylation of Large Aromatics and the Role of Defects. Microporous Mesoporous Mater. 129, 319–329 (2010). [↩]
- Sharma, D.; Rasaily, S.; Pradhan, S.; Baruah, K.; Tamang, S.; Pariyar, A. HKUST-1 Metal Organic Framework as an Efficient Dual-Function Catalyst: Aziridination and One-Pot Ring-Opening Transformation for Formation of β-Aryl Sulfonamides with C–C, C–N, C–S, and C–O Bonds. Inorg. Chem. 60, 7794–7802 (2021). [↩]
- Zelenka, T.; Simanova, K.; Saini, R.; Zelenkova, G.; Nehra, S. P.; Sharma, A.; Almasi, M. Carbon Dioxide and Hydrogen Adsorption Study on Surface-Modified HKUST-1 with Diamine/Triamine. Sci. Rep. 12 (2021). [↩] [↩] [↩] [↩] [↩] [↩] [↩]
- Hendon, C. H.; Walsh, A. Chemical Principles Underpinning the Performance of the Metal–Organic Framework HKUST-1. Chem. Sci. 6, 3674–3683 (2015). [↩] [↩]
- Zhang, Y.; Wang, H.; Li, X.; Chen, J.; Liu, Z. A techno-economic analysis of carbon capture and utilization for methanol production. Carbon Research. 2, 100060 (2023). [↩]
- Dong, H.; Li, L.; Li, C. Controlled Alkali Etching of MOFs with Secondary Building Units for Low-Concentration CO₂ Capture. Chem. Sci. 14, 8507–8513 (2023). [↩]
- Ryu, U.; Jee, S.; Rao, P. C.; Shin, J.; Ko, C.; Yoon, M.; Park, K. S.; Choi, K. M. Recent Advances in Process Engineering and Upcoming Applications of Metal–Organic Frameworks. Coord. Chem. Rev. 426, 213544 (2020). [↩]
- Gargiulo, V.; Raganati, F.; Ammendola, P.; Alfe, M.; Chirone, R. HKUST-1 Metal Organic Framework as CO₂ Adsorbent in a Sound Assisted Fluidized Bed. Chem. Eng. Trans. 43, 1087–1092 (2015). [↩]
- Helios SciTech. HKUST-1 (MOF-199), Cu-BTC Metal–Organic Framework. https://heliosscitech.com/product/hkust-1-mof-199-helios/ [↩]
- Takeda, H.; Kamiyama, H.; Okamoto, K.; Irimajiri, M.; Mizutani, T.; Koike, K.; Sekine, A.; Ishitani, O. Highly Efficient and Robust Photocatalytic Systems for CO₂ Reduction Consisting of a Cu(I) Photosensitizer and Mn(I) Catalysts. J. Am. Chem. Soc. 140, 17241–17254 (2018). [↩]
- Tracking Clean Energy Progress 2023. https://www.iea.org/reports/tracking-clean-energy-progress-2023 [↩]






