Abstract
Plastic pollution has more than doubled since the turn of the century, with 20 million metric tons of plastic waste entering the environment each year. Plastics are extremely durable materials that require hundreds of years to decompose fully. We investigate pro-oxidant and transition metal complex additives as potential facilitators to plastic biodegradation. Pro-oxidant additives like cobalt (II) stearate engage oxidation-reduction reactions in plastics to weaken bonds, while starch additives naturally decompose, leaving large gaps that weaken the polymer structures of plastics. We co-extruded cobalt (II) stearate and potato starch with three polymers – PETg, HDPE, and PLA- and incubated them in a bacteria-rich environment for 21 days under ideal conditions for microorganism growth (37°C). We then analyze our data with a Mann-Whitney U test. Cobalt (II) stearate demonstrated a significant correlation with increased degradation in PLA and potato starch demonstrated a significant correlation with increased degradation in PETg and HDPE. These results suggest that starch and pro-oxidant additives could enhance the degradation of biodegradable plastics similar in nature to PLA while starch additives are more compatible with nonbiodegradable plastics similar in nature to PETg and HDPE. The additives researched in this study can be utilized in the manufacturing of single-use plastics to combat the growing threat of plastic pollution.
Keywords: plastics, pro-oxidant, transition metal complex, starch, polymers, biodegradation, additives
Introduction
Plastics consist of large molecules known as polymers, which are formed by repeatedly linking smaller chemical units known as monomers, typically of a single species, through strong covalent bonds1,2. Plastics have found widespread use for their low-cost and tunable material properties. For example, poly(ethylene terephthalate glycol) (PETg) is frequently used in manufacturing and packaging and is infused with glycol, a food source for microorganisms, to increase its flexibility3. High-density polyethylene (HDPE) is commonly used in household products for its durability4. Poly(lactic acid) (PLA) is a biodegradable polymer often used in 3D printing5,6. Additives are commonly mixed into polymers to enhance certain properties such as color, strength, or stability. These additives are added during extrusion, a process in which the polymer and additives are blended at high temperatures and pressure7. The stability of C-C bonds, combined with the high molecular weights of most commodity plastics, results in extremely slow degradation for most plastics8,9. Many plastics have half-lives of hundreds of years, which combined with the 175 Megatons of plastic entering landfills and the environment each year, raises a major environmental concern10. For instance, nearly 700 marine species have been directly harmed by plastic waste through ingestion, entanglement, and smothering11.
One potential additive that can accelerate plastic degradation is starch. Starch is an inexpensive, organic additive that can be blended with polymers to create bioplastics, defined in this paper as biodegradable plastics derived from renewable resources12. Starch additives are filler additives that have been found to significantly enhance mechanical properties of plastics like tensile strength and elongation at 30 wt. %13. When blended with a polymer, the starch inside the plastic is naturally degraded by microorganisms, leaving behind a less dense polymer structure that is then easier to biodegrade via natural processes such as thermal oxidation14,15. Starch is composed of two types of polysaccharides, amylose and amylopectin. While amylopectin is the more biodegradable of the two components, amylose typically results in higher plastic strength and ductility16. Thus, the ratio of amylose to amylopectin must be considered when incorporating starch into plastic blends to ensure an effective combination of strength and biodegradability. For example, potato starch, which is rich in amylopectin while still containing a significant percentage of amylose, is an effective starch for bioplastics17. However, because most commodity plastics are hydrophobic and starches are hydrophilic, starch-based plastics often result in weakened properties concerning interactions with water.
Another type of additive that could potentially accelerate the degradation of plastics is transition metal complexes. Being chemical additives, they are typically incorporated into plastic blends up to 5 wt. %18. Transition metal complexes represent a category of pro-oxidants that may also be useful in improving the biodegradation of polymers through a series of oxidation-reduction reactions19,20,21. In a process known as thermal degradation, which begins at 28°C-150°C, heated polymers with transition metal complexes can be broken down during exposure to atmospheric oxygen into small fragments that have increased hydrophilicity and become more susceptible to forms of biotic degradation22’23. Thermal degradation can be accelerated by combining plastics with microorganisms in organic compost material that catalyzes the process24. Additionally, the process of composting generates heat25, which further accelerates plastic degradation. For example, cobalt (II) stearate is a representative transition metal pro-oxidant compound that is recognized to enable thermal degradation26’27.
Although potato starch and cobalt (II) stearate could theoretically catalyze the degradation of many different polymers14,15,26,20,21, the rate at which they can enhance biodegradation and the variability of this effect between different polymeric materials is largely unknown. This study aims to address gaps in knowledge of the latter, which is pertinent for commercial purposes in determining which additives induce optimal degradation in certain plastics.
It should be noted that we were constricted by our low sample size to expand the research to a large enough sample for the central limit theorem to be utilized for a standard distribution. Even for the Mann-Whitney U test, an ideal comparison would occur with a sample size of n ≥ 5. Thus, we hope that future works can improve upon our results by conducting a similar-natured experiment with a greater number of trials.
In this study, we perform a comparative analysis of cobalt (II) stearate and starch in accelerating the biodegradation rate across polymer classes. Plastic blends are first created by extruding various polymers with starch and pro-oxidant additives, incorporated in concentrations commonly used and found to benefit plastic properties in manufacturing and commercial applications (30.0 wt. % and 5.0 wt. %, respectively)28,29. Then, we track the biodegradation rates of these plastic blends in a carefully controlled environment designed to mimic ideal conditions for degradation. Following a statistical analysis of our results, we investigate potential applications of our findings and provide recommendations for how they can be employed.
We hypothesize that due to the inherently biodegradable nature of both potato starch and PLA, the energy provided to microbial colonies in the depolymerization of potato starch will enable an accelerated secretion of hydrolytic enzymes to break down the polymer matrix of PLA to a greater extent than resistant polymers with higher incompatibility with the additives such as HDPE and PETg30,31 Meanwhile, for shorter testing periods, cobalt (II) stearate could potentially serve as a less-effective oxidizing agent, particularly for non-biodegradable polymers such as HDPE and PETg, because the chemical, thermal oxo-degradative mechanism by which it facilitates degradation may occur less rapidly compared to the physical mechanism of filler, starch-based additives like potato starch. Alternative results may occur with extended testing periods in further study.
Hence, we speculate that our results will have greater statistical significance for potato starch and biodegradable compound blends and less statistical significance for cobalt (II) stearate and non-biodegradable compound blends. Specifically, we expect the PLA-starch blend to degrade the most, followed by the PETg-starch blend, PLA-stearate blend, PETg-stearate blend, HDPE-starch blend, and finally the HDPE-stearate blend.
Methods
The nature of this study is an experiment, testing the effects of cobalt (II) stearate and potato starch additives on the masses of polymers over time, or effectively, the degradation rate of polymers.
Sample Preparation
PETg (Eastar copolyester 6763 PETg pellets), HDPE (VViViD), PLA (Ingeo 4032D), cobalt (II) stearate (AK Scientific, Inc.), and potato starch (Bob’s Red Mill) were used as received. Degradation of plastic blends with cobalt (II) stearate and potato starch was studied in three polymers: polyethylene terephthalate glycol (PETg), high-density polyethylene (HDPE), and poly(lactic acid) (PLA). HDPE is one of the most common polymers used in commercial products, with tens of millions of tons being produced each year32. Additionally, PETg and HDPE are notably non-biodegradable, whereas PLA is industrially compostable33’34.
We co-extruded nine different plastic blends (5.00 g total each) using a ThermoHaake Mini Lab II twin screw extruder: three polymer controls (100 wt. % PETg, HDPE, PLA), three cobalt (II) stearate blends, and three potato starch blends. To ensure the viability of our findings for commercial applications, we selected the quantity of additive added based on commercial findings and the degradation-facilitating mechanism of each additive. Because pro-oxidant additives, being chemical additives that operate by chemical oxidation-reduction reactions, are commonly incorporated in concentrations of 5.0 wt. % in commercial applications, we blended 5.0 wt. % of cobalt (II) stearate with each polymer to test the effects of the additive35. Since potato starch acts as a filler additive and has been found to enhance plastic mechanical properties at 30.0 wt%, we added it in a quantity of 30.0 wt. %28. We co-extruded each PETg blend at 230oC, each HDPE blend at 190C, and each PLA blend at 220C. Potato starch and cobalt (II) stearate are both thermally stable up to 250oC36’37. We set the extruder to run at 100 rotations per minute (RPM).
Degradation Testing
We created an environment for plastic degradation through the use of organic matter compost as a source of degradative microorganisms. We heated the blends in this compost in an incubator (Beokal Inc. Incubator Model 133000) to accelerate the degradative mechanisms of additives and induce further microorganism growth. For each plastic blend, we combined 15.00 g of our composting mixture with 0.25-0.52 g of the extruded plastic in a Petri dish (90 mm diameter x 15 mm depth). We repeated this 3 times for each plastic blend, totaling to 3 trials per mixture and 27 total trials. Our compost had a moisture content of 0.7723 g of water per g of compost (77.23%), with a water holding capacity (WHC) of 0.404 g of water per gram of compost. The compost status was mature and had a pH of 6.5 and an oxygen availability that was low but not anoxic. The WHC was found using the funnel, filter paper, and drainage (MWHCFFPD) method38. We incubated the Petri dishes at ~37°C for 21 days. Although thermal degradation begins at 28°C -150°C39, we ran our experiment at the lower end of that range (37°C), the optimal temperature for the growth of mesophilic bacteria40, to ensure that mesophilic bacteria can grow simultaneously.
Additionally, considering the biotic degradation mechanisms of starch we studied, we controlled against potential abiotic degradation of starch during the testing process. We enclosed our samples indoors in a closed incubator without exposure to sunlight, preventing UV rays from inducing abiotic photooxidation in the starch-based plastics41. This did not impact the biotic degradation of starch by microorganisms and the abiotic, thermal oxidative degradation mechanisms of pro-oxidant-based plastics, as they are independent of UV availability42,22,43. We also controlled for the pH of the compost environment. At pH = 6.5, abiotic degradation of starch is restricted while biotic degradation of starch and abiotic, thermal oxidative degradation mechanisms of pro-oxidant-based plastics are facilitated44,45,22. As outlined previously, to maintain optimal conditions for biotic degradation of starch-based plastics and abiotic degradation of our pro-oxidant-based plastics, we incubated our samples at ideal temperature conditions of 37°C.
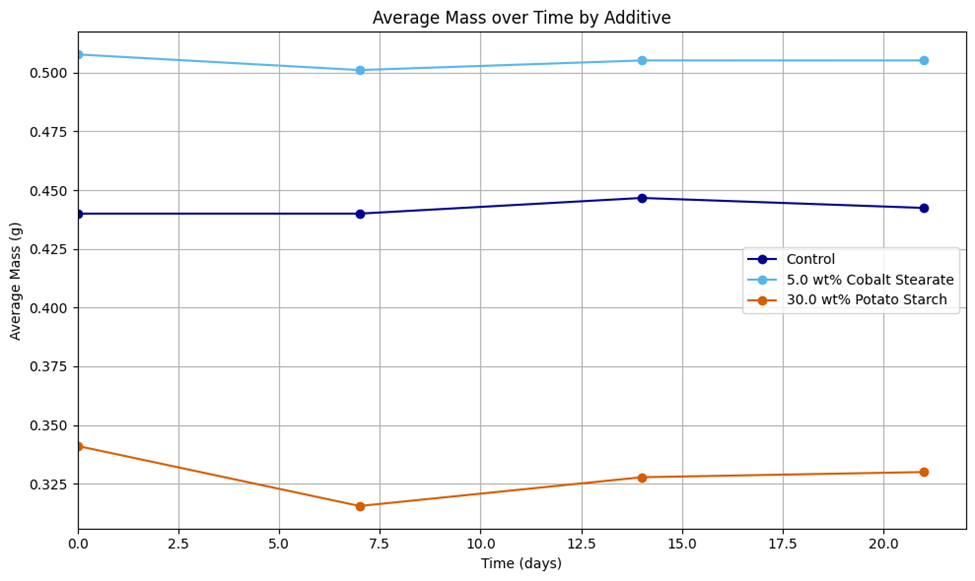
We compared the degradation in each blend over time, where degradation was quantified as the percent change in mass after degradation testing. After cleaning the samples to isolate the plastic, we recorded the masses of each plastic blend independently (using a Flinn Scientific electronic balance OB2090 x 0.01 g) and recorded qualitative observations prior to biodegradation testing and at 7, 14, and 21 days.
Statistical Analysis
We collected the data and interpreted its significance using statistical analysis (software) by a Mann-Whitney U test. A Mann-Whitney U test is used to determine the significance of degradation induced by an additive-polymer blend. We also used Microsoft Excel to plot our recorded trends in degradation.
Results
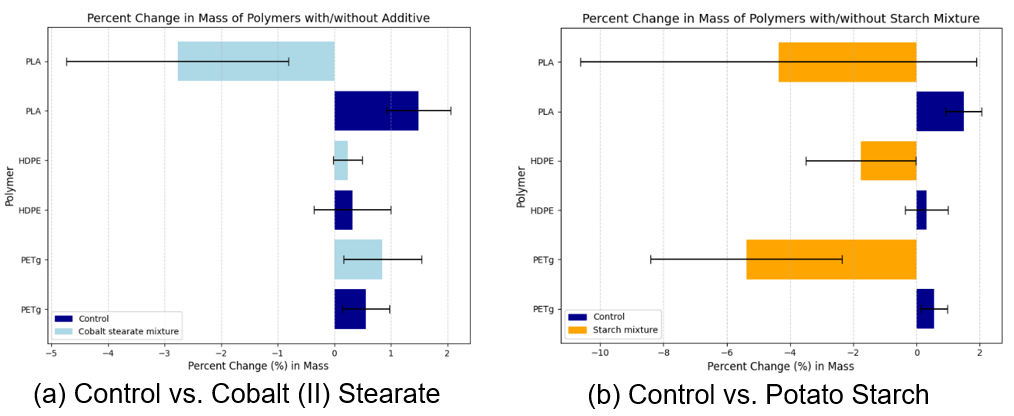
The quantitative results are summarized below:
| Additive-Polymer Blend | Percent Change in Mass (Median ± 95% CI) |
| 100 wt. % PETg (control) | 0.56±0.42 |
| 100 wt. % HDPE (control) | 0.32±0.68 |
| 100 wt. % PLA (control) | 1.49±0.57 |
| PETg + 5.0 wt. % cobalt (II) stearate | 0.85±0.69 |
| HDPE + 5.0 wt. % cobalt (II) stearate | 0.24±0.26 |
| PLA + 5.0 wt. % cobalt (II) stearate | -2.77±1.96 |
| PETg + 30.0 wt. % potato starch | -5.38±3.03 |
| HDPE + 30.0 wt. % potato starch | -1.76±1.74 |
| PLA + 30.0 wt. % potato starch | -4.36±6.26 |
Quantitative Analysis
Summary Table of p-values
The tables below summarize the p-values calculated for each additive in testing the hypothesis that the additive significantly accelerates the polymer’s degradation:
| Additive-Polymer Blend | |
| PETg w/ 5.0 wt. % cobalt (II) stearate | 0.90 |
| HDPE w/ 5.0 wt. % cobalt (II) stearate | 0.50 |
| PLA w/ 5.0 wt. % cobalt (II) stearate | 0.04 |
| PETg w/ 30.0 wt. % potato starch | 0.05 |
| HDPE w/ 30.0 wt. % potato starch | 0.05 |
| PLA w/ 30.0 wt. % potato starch | 0.33 |
The p-values for PLA + 5.0 wt. % cobalt (II) stearate, PETg + 30.0 wt. % potato starch, and HDPE + 30.0 wt. % potato starch are less than or equal to the critical value for α = 0.05. Thus, we reject the null hypothesis that the additive does not increase the mean mass reduction of plastics for those blends (Table 2). For all other blends in this experiment, we fail to reject the null hypothesis.
Summary of Significance of Results
Below are the statistical significance of our findings according to the Mann-Whitney U test:
| Polymer | Acceleration of Degradation |
| PETg | Insignificant |
| HDPE | Insignificant |
| PLA | Significant |
There is significant evidence that 5.0 wt. % cobalt (II) stearate accelerates the biodegradation of PLA. There is not significant evidence that 5.0 wt. % cobalt (II) stearate accelerates the biodegradation of PETg or HDPE.
| Polymer | Acceleration of Degradation |
| PETg | Significant |
| HDPE | Significant |
| PLA | Insignificant |
There is significant evidence that 30.0 wt. % potato starch accelerates the biodegradation of PETg. There is also significant evidence that 30.0 wt. % potato starch accelerates the biodegradation of HDPE. There is not significant evidence that 30.0 wt. % potato starch accelerates the biodegradation of PLA.
In the discussion of these findings, we will investigate applications of the additives and make recommendations based on the polymers we found our additives to most benefit compared to the general utility and abundance of that additive.
Qualitative Observations
Finally, we share our most significant qualitative observations from the data collection process for those who intend to recreate or modify our study:
| Plastic extrusion day | Initial observations for incubator setup and plastic blends |
| The cobalt (II) stearate blends melted faster than the potato starch blends. The PLA + cobalt (II) stearate blend had the quickest melting speed. The PETg + potato starch blend had a slower melting speed and spent more time in the extruder; it could be more oxidized. | The control blends had a whiter hue, the cobalt (II) stearate blends were purple, and the potato starch blends were a tan color. The cobalt (II) stearate blends were smoother, while the starch blends were coarse. The starch blends and HDPE + cobalt (II) stearate blend were more flexible. |
| Initial data collection | Final data collection after 21 days |
| The organic compost was further shriveled and had turned a darker hue. The plastics seemed to have maintained the same size and shape. | Most of the transparent plastics had turned a dark yellow-brown hue. |
Discussion
Based on the results, it can be concluded that pro-oxidant additives such as cobalt (II) stearate act as a more effective additive than starches like potato starch in facilitating biodegradation of non-biodegradable plastics such as HDPE and PETg. In contrast, it can be concluded that starch is more effective than pro-oxidant additives in facilitating the biodegradation of biodegradable plastics such as PLA. Cobalt (II) stearate demonstrated significant acceleration only of the degradation of PLA, while potato starch demonstrated significant acceleration only of the degradation of HDPE and PETg (Table 3, Table 4). This disagrees with our hypothesis that potato starch would be most effective with PLA due to the polymer’s inherent biodegradable nature being accelerated by the decomposition of potato starch and that cobalt (II) stearate would be less effective than potato starch due to the slower nature by which thermal degradation occurs. We speculate that this could be due to cobalt (II) stearate accelerating pre-existing chemical mechanisms of biodegradation operating on polymer bonds, rather than initiating a pathway for biodegradation like starch additives do. Additionally, starch creates gaps in the structural matrix of PETg and HDPE that results in microbial attack, which may accelerate degradation of those polymers to a greater extent than in PLA, in which this process is already occurring due to its biodegradable nature.
Additionally, a limitation of our study was the relatively small sample size of our plastics. Due to the lengthy extrusion time for each blend and a time constraint that was placed on our research, we were limited to 27 total blends, with 3 blends for each polymer/additive blend. This made it necessary for us to employ a rank test for statistical significance that didn’t depend on a large sample size, potentially decreasing the power of our results. We hope that future researchers can expand on our work by using it as a guideline and increasing the sample size in order to reach a more well-supported conclusion.
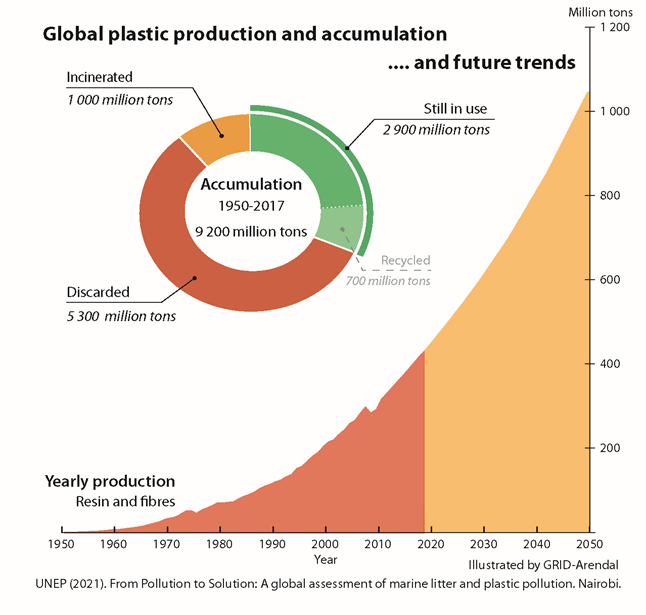
The world currently produces around 460 million metric tons of plastic each year, around 20 million of which end up as waste in the environment47. This trend continues to grow upwards, as plastics become more and more integral to modern society. In fact, annual plastic production is expected to nearly double by the year 2050 (Figure 6). We discovered that compared to pro-oxidant additives, starch additives are more effective for plastic biodegradation in common polymers like PETg, HDPE, and PLA. By shifting to the use of these additives, plastic waste can biodegrade more quickly and help mitigate the ever-growing plastic pollution problem.
Future Work
Future works could also vary the concentrations of additive added to each blend, allowing for a comparison to be made between various concentrations. These would both serve to expand upon our limited sample size and provide more accurate data on how the concentration of the additive affects the degradation of the plastic. Additionally, future studies could incorporate new methods such as measuring gas evolution or performing infrared spectroscopy to further test the level of degradation of each plastic. Furthermore, additional studies on mechanical properties of these plastic blends such as tensile strength or elasticity would be helpful for providing more accurate recommendations for their industrial applications.
Our work has significant applications in the plastic manufacturing and packaging industries. By shifting to using starch-based plastics in packaging products, we can ensure a lower biodegradation period for any of these plastics that end up as environmental waste. Particularly, due to PLA’s affinity with cobalt (II) stearate, our findings could have significant applications in 3D printing. PLA is the most commonly used 3D printing filament48, and by accelerating its biodegradation, we can ensure that less of the PLA manufactured ends up in the environment. This enhanced biodegradation is especially beneficial as PLA is non-recyclable in most commercial facilities49. Additionally, the red color of the cobalt (II) stearate + PLA blend introduces possible cosmetic uses for cobalt (II) stearate as an additive.
References
- J. A. Brydson. Plastics materials. (1999). [↩]
- B. Björkner, M. Frick-Engfeldt, A. Pontén, E. Zimerson. Plastic materials. Contact dermatitis. 695–728 (2011). [↩]
- C. Yan, C. Kleiner, A. Tabigue, V. Shah, G. Sacks, D. Shah, et al. PETG: Applications in modern medicine. Engineered Regeneration. 5.1, 45–55 (2024). [↩]
- A. H. Awad, R. El Gamasy, A/ Abd El Wahab, M. H. Abdellatif. Mechanical and physical properties of PP and HDPE. Eng Sci. 4.2, 34 (2019). [↩]
- K. M. Nampoothiri, N. R. Nair, R. P. John. An overview of the recent developments in polylactide (PLA) research. Bioresource technology. 101.22, 8493–8501 (2010). [↩]
- S. Farah, D. G. Anderson, R. Langer. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Advanced drug delivery reviews. 107, 367–392 (2016). [↩]
- G. Pritchard. Plastics additives: An AZ reference. 1 (2012). [↩]
- A. A. Shah, F. Hasan, A. Hameed, S. Ahmed. Biological degradation of plastics: A comprehensive review. Biotechnology advances. 26.3, 246–265 (2008). [↩]
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, et al. Degradation rates of plastics in the environment. ACS Sustainable Chemistry & Engineering. 8.9, 3494–3511 (2020). [↩]
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, et al. Degradation rates of plastics in the environment. ACS Sustainable Chemistry & Engineering. 8.9, 3494–3511 (2020). [↩]
- S. C. Gall, R. C. Thompson. The impact of debris on marine life. Marine pollution bulletin. 92.1-2,170–179 (2015). [↩]
- J. Gonzalez-Gutierrez, P. Partal, M. Garcia-Morales, C. Gallegos. Development of highly-transparent protein/starch-based bioplastics. Bioresource technology. 101.6, 2007-2013 (2010). [↩]
- K. Oduola. Effect of Starch on the Mechanical and Rheological Properties of Polypropylene. American Journal of Chemical Engineering. 3 (2015). [↩]
- S. Jayarathna, M. Andersson, R. Andersson. Recent advances in starch-based blends and composites for bioplastics applications. Polymers. 14.21, 4557 (2022). [↩] [↩]
- R. Anugrahwidya, B. Armynah, D. Tahir. Bioplastics starch-based with additional fiber and nanoparticle: Characteristics and biodegradation performance: A review. Journal of Polymers and the Environment. 29.11, 3459–3476 (2021). [↩] [↩]
- C. Chaléat, P. J. Halley, R. Truss. Mechanical properties of starch-based plastics. In: Starch polymers. 187–209 (2014). [↩]
- J. Jane, A. Xu, M. Radosavljevic, P. Seib. Location of amylose in normal starch granules. i. Susceptibility of Amylose and Amylopectin to Cross-Linking Reagents. 69, 405–409 (1992). [↩]
- F. Sciscione, H. Hailes, M. Miodownik. The performance and environmental impact of pro-oxidant additive containing plastics in the open unmanaged environment—a review of the evidence. Royal Society Open Science. (2023). [↩]
- R. Banasz, M. Wałęsa-Chorab. Polymeric complexes of transition metal ions as electrochromic materials: Synthesis and properties. Coordination Chemistry Reviews. 389, 1–18 (2019). [↩]
- S. Fontanella, S. Bonhomme, M. Koutny, L. Husarova, J. M. Brusson, J. P. Courdavault, et al. Comparison of the biodegradability of various polyethylene films containing pro-oxidant additives. Polymer degradation and stability. 95.6, 1011–1021 (2010). [↩] [↩]
- L. Contat-Rodrigo. Thermal characterization of the oxo-degradation of polypropylene containing a pro-oxidant/pro-degradant additive. Polymer degradation and stability. 98.11, 2117–2124 (2013). [↩] [↩]
- R. Asriza, I. Arcana, V. Fabiani. Thermal degradation of high-density polyethylene containing cobalt stearat as oxidant additive. In: IOP conference series: Earth and environmental science. 353, 012036 (2019). [↩] [↩] [↩]
- R. O. Asriza, I. Arcana. Synthesis of cobalt stearate as oxidant additive for oxo-biodegradable polyethylene. In: AIP conference proceedings. 1677 (2015). [↩]
- R. O. Asriza, I. Arcana. Synthesis of cobalt stearate as oxidant additive for oxo-biodegradable polyethylene. In: AIP conference proceedings. 1677 (2015). [↩]
- S. Fan, A. Li, A. ter Heijne, C. J. Buisman, W. S. Chen. Heat potential, generation, recovery and utilization from composting: A review. Resources, Conservation and Recycling. 175, 105850 (2021). [↩]
- R. Banasz, M. Wałęsa-Chorab. Polymeric complexes of transition metal ions as electrochromic materials: Synthesis and properties. Coordination Chemistry Reviews. 389, 1–18 (2019). [↩] [↩]
- R. O. Asriza, I. Arcana. Synthesis of cobalt stearate as oxidant additive for oxo-biodegradable polyethylene. In: AIP conference proceedings. 1677 (2015). [↩]
- K. Oduola. Effect of Starch on the Mechanical and Rheological Properties of Polypropylene. American Journal of Chemical Engineering. 3 (2015). [↩] [↩]
- F. Sciscione, H. Hailes, M. Miodownik. The performance and environmental impact of pro-oxidant additive containing plastics in the open unmanaged environment—a review of the evidence. Royal Society Open Science. (2023). [↩]
- S. S. Zamir, B. Fathi, A. Ajji, M. Robert, S. Elkoun. Biodegradability assessment of HDPE-based biocomposites: Influence of starch and fiber composition. Materials Today Communications. 40, 109786 (2024). [↩]
- M. Zighead, B. Benotmane, H. Ferkous, N. Ramdane, A. Boublia, M. Ahmed, A. Bourbia, S. Lemboub, K. K. Yadav, Y.
,Benguerba. Biodegradation of modified starch/poly lactic acid nanocomposite in soil. Polymer degradation and stability. 199, 109902 (2022). [↩] - RA Markets. Cision PR Newswire. (2017). [↩]
- A. Muthukumar, S. Veerappapillai. Biodegradation of plastics–a brief review. Int J Pharm Sci Rev Res. 31.2, 204–209 (2015). [↩]
- T. Polygenis. PETG vs PLA: Differences and comparison. Wevolver. https://www.wevolver. com/article/pet g-vs-pla-how-do-they-compare (2023). [↩]
- F Sciscione, H Hailes, M Miodownik. The performance and environmental impact of pro-oxidant additive containing plastics in the open unmanaged environment—a review of the evidence. Royal Society Open Science. (2023). [↩]
- P. Dony, F. Berzin. Thermogravimetric, Morphological and Infrared Analysis of Blends Involving Thermoplastic Starch and Poly(ethylene-co-methacrylic acid_ and Its Ionomer Form. Molecules. 28, 4519 (2023). [↩]
- Cobalt(II) Stearate. BenchChem. https://www.benchchem.com/product/b085634 (2025). [↩]
- J. T. Nelson, T. A. Adjuik, E. B. Moore, A. D. VanLoocke, A. R. Reyes, M. D. McDaniel. A Simple, Affordable, Do-It-Yourself Method for Measuring Soil Maximum Water Holding Capacity. Communications in Soil Science and Plant Analysis. 55, 1190-1204 (2023). [↩]
- R. G. Chaudhary, P Ali, NV Gandhare, JA Tanna, HD Juneja. Thermal decomposition kinetics of som36e transition metal coordination polymers of fumaroyl bis (paramethoxyphenylcarbamide) using DTG/DTA techniques. Arabian Journal of Chemistry. 12.7, 1070–1082 (2019). [↩]
- M. Zwietering, J. De Koos, B. Hasenack, J. De Witt, K. Van’t Riet. Modeling of bacterial growth as a function of temperature. Applied and environmental microbiology. 57.4, 1094–1101 (1991). [↩]
- E. Yousif, R Haddad. Photodegradation and photostabilization of polymers, especially polystyrene: review. SpringerPlus. 2, 398 (2013). [↩]
- S. Courbier. Breaking Dawn: The twilight of starch degradation in the light. Plant Physiology. 189, 1887-1889 (2022). [↩]
- M. Kurdziel, M. Labanowska, S. Pietrzyk, P. Pajak, K. Krolikowska, A. Szwengiel. The effect of UV-B irradiation on structural and functional properties of corn and potato starches and their components. Carbohydrate Polymers. 289 (2022). [↩]
- M. E. Apostolidi, S. Kalantzi, D. G. Hatzinikolaou, D. Kekos, D. Mamma. Catalytic and thermodynamic properties of an acidic α-amylase produced by the fungus Paecilomyces variotii ATHUM 8891. 3 Biotech. 10, 311 (2020). [↩]
- S. Striegler. Developing Catalysts for the Hydrolysis of Glycosidic Bonds in Oligosaccharides Using a Spectrophotometric Screening Assay. 14, 12940-12946 (2024). [↩]
- United Nations Environment Programme. From pollution to solution: A global assessment of marine litter and plastic pollution. (2021). [↩]
- IUCN. Plastic pollution. https://iucn.org/resources/issues-brief/plastic-pollution (2024). [↩]
- J. Horvath, R. Cameron. Mastering 3D printing: A guide to modeling, printing, and prototyping. (2020). [↩]
- E. Moreno, F. R. Beltrán, M. P. Arrieta, G. Gaspar, L. M. Muneta, R. Carrasco-Gallego, S. Yáñez, D. Hidalgo-Carvajal, M. U. de la Orden, J. M. Urreaga. Technical evaluation of mechanical recycling of PLA 3D printing wastes. In: Proceedings. 69, 19 (2020). [↩]